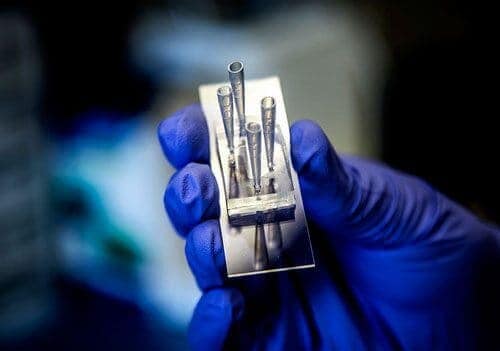
University of Michigan researchers have used a “kidney on a chip” device to mimic the flow of medication through human kidneys and measure its effect on kidney cells.
The new technique could lead to more precise dosing of drugs, including some potentially toxic medicines often delivered in intensive care units.
Precise dosing in intensive care units is critical, as up to two-thirds of patients in the ICU experience serious kidney injury. Medications contribute to this injury in more than 20 percent of cases, largely because many intensive care drugs are potentially dangerous to the kidneys.
Determining a safe dosage, however, can be surprisingly difficult. Today, doctors and drug developers rely mainly on animal testing to measure the toxicity of drugs and determine safe doses. But animals process medications more quickly than humans, making it difficult to interpret test results and sometimes leading researchers to underestimate toxicity.
The new technique offers a more accurate way to test medications, closely replicating the environment inside a human kidney. It uses a microfluidic chip device to deliver a precise flow of medication across cultured kidney cells. This is believed to be the first time such a device has been used to study how a medication behaves in the body over time, called its “pharmacokinetic profile.”
“When you administer a drug, its concentration goes up quickly and it’s gradually filtered out as it flows through the kidneys,” said Shuichi Takayama, U-M professor of biomedical engineering. “A kidney on a chip enables us to simulate that filtering process, providing a much more accurate way to study how medications behave in the body.”
Takayama said the use of an artificial device provides the opportunity to run test after test in a controlled environment. It also enables researchers to alter the flow through the device to simulate varying levels of kidney function.
“Even the same dose of the same drug can have very different effects on the kidneys and other organs, depending on how it’s administered,” said former U-M researcher Sejoong Kim, an associate professor at Korea’s Seoul National University Bundang Hospital. “This device provides a uniform, inexpensive way to capture data that more accurately reflects actual human patients.”
In the study, the team tested their approach by comparing two different dosing regimens for gentamicin, an antibiotic that’s commonly used in intensive care units. They used a microfluidic device that sandwiches a thin, permeable polyester membrane and a layer of cultured kidney cells between top and bottom compartments.
They then pumped a gentamicin solution into the top compartment, where it gradually filtered through the cells and the membrane, simulating the flow of medication through a human kidney. One test started with a high concentration that quickly tapered off, mimicking a once-daily drug dose. The other test simulated a slow infusion of the drug, using a lower concentration that stayed constant. Takayama’s team then measured damage to the kidney cells inside the device.
They found that a once-daily dose of the medication is significantly less harmful than a continuous infusion—even though both cases ultimately delivered the same dose of medication. The results of the test could help doctors better optimize dosing regimens for gentamicin in the future. Perhaps most importantly, they showed that a kidney on a chip device can be used to study the flow of medication through human organs.
“We were able to get results that better relate to human physiology, at least in terms of dosing effects, than what’s currently possible to obtain from common animal tests,” Takayama said. “The goal for the future is to improve these devices to the point where we’re able to see exactly how a medication affects the body from moment to moment, in real time.”
Takayama said the techniques used in the study should be generalizable to a wide variety of other organs and medications, enabling researchers to gather detailed information on how medications affect the heart, liver and other organs. In addition to helping researchers fine-tune drug dosing regimens, he believes the technique could also help drug makers test drugs more efficiently, bringing new medications to market faster.
Within a few years, Takayama envisions the creation of integrated devices that can quickly test multiple medication regimens and deliver a wide variety of information on how they affect human organs. PHASIQ, an Ann Arbor-based spinoff company founded by Takayama in conjunction with the U-M Office of Technology Transfer, is commercializing the biomarker readout aspect of this type of technology.
The study, published in the journal Biofabrication, is titled “Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip.” Funding and assistance for the project was provided by the National Institutes of Health (grant number GM096040), U-M Center for Integrative Research in Critical Care, U-M Biointerfaces Institute, National Research Foundation of Korea and Korean Association of Internal Medicine Research Grant 2015.