Women’s decreased ability to produce healthy eggs as they become older may be due to excessive scarring and inflammation in their ovaries, reports a new Northwestern Medicine study in mice.
This is the first study to show the ovarian environment ages and that aging affects the quality of eggs it produces. These findings could result in new treatments that preserve fertility by delaying ovarian aging.
Most reproductive research focuses on women’s eggs and trying to understand why their number and quality deteriorate as a woman enters her forties. Deteriorating eggs contribute to infertility, miscarriages and birth defects.
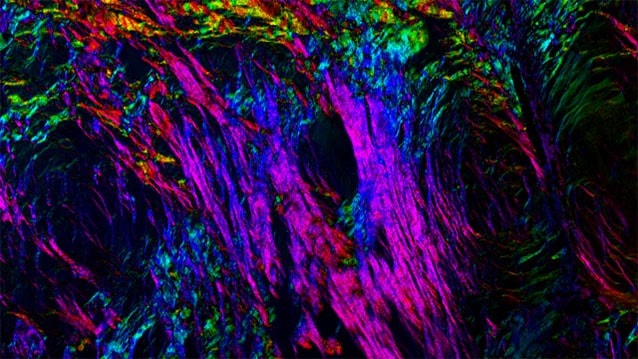
But in this study scientists examined the reproductive age-related changes that occur in the environment in which the eggs develop, known as the ovarian stroma. The environment in which cells grow and develop can greatly influence their quality and function, but surprisingly little is known about how the ovarian stroma changes with age.
“Under the microscope, eggs from reproductively young and old animals may look identical, but the environment in which they are growing is completely different,” said lead study author Francesca Duncan, executive director of the Center for Reproductive Science at Northwestern University Feinberg School of Medicine. “Ovaries from reproductively old mice are fibrotic and inflamed. There is no way this environment won’t impact the eggs growing in it, and it very likely contributes to their decrease in quality.”
The study was published August 5 in the journal Reproduction. Duncan led the study when she was at the University of Kansas Medical Center.
In this study, researchers analyzed ovarian tissue from populations of reproductively “young” (equivalent to women in their early twenties) and “old” mice (equivalent to women ages 38-45). They consistently identified fibrosis in the reproductively “old” mice. This age period is associated with a decline in reproductive function and egg quality in both humans and mice. In some reproductively “old” mice, up to 35 percent of the ovarian tissue was fibrotic.
Researchers also found a type of immune cell (multinucleated macrophage giant cells) in the ovaries of reproductively “old” mice only. When found in other tissues, these cells are associated with chronic inflammation. They also found ovaries from mice of advanced reproductive age expressed genes and produced proteins that are highly inflammatory.
“Our work establishes fibrosis and inflammation as hallmarks of the aging ovary and lays the foundation for considering the use of anti-fibrotic or anti-inflammatory treatments to delay or counteract the impact of reproductive aging,” said Duncan, also an associate research professor in obstetrics and gynecology at Feinberg.
“People tend to overlook that the egg is growing in a complex environment, and no one has taken a deep look at what is happening to that environment with age,” Duncan added. “It’s an underappreciated area.”
The findings have broader implications for women’s health because ovarian fibrosis is a key feature of polycystic ovary syndrome, a common endocrine system disorder among women of reproductive age, and is also a consequence of chemotherapy and radiation, Duncan said.
Duncan’s research team is currently investigating how to therapeutically target the ovarian environment to improve reproductive function.
This work was supported by the Center for Reproductive Health After Disease (P50 HD076188), the National Centers for Translational Research in Reproduction and Infertility, the Centers of Biomedical Research Excellence (P20 GM104936), the National Center for Research Resources (P20 RR021940), the National Institute of General Medical Sciences (P20 GM103549) and the National Institute of Environmental Health Sciences ‘Training Program in Environmental Toxicology’ (T32 ES007079) of the National Institutes of Health.