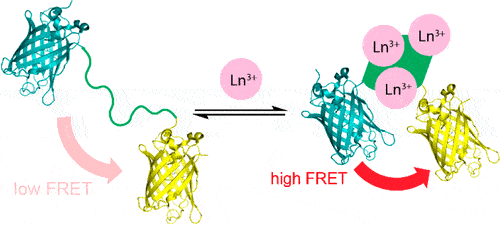
A more efficient and cost-effective way to detect lanthanides, the rare earth metals used in smartphones and other technologies, could be possible with a new protein-based sensor that changes its fluorescence when it binds to these metals. A team of researchers from Penn State developed the sensor from a protein they recently described and subsequently used it to explore the biology of bacteria that use lanthanides. A study describing the sensor appears online in the Journal of the American Chemical Society.
“Lanthanides are used in a variety of current technologies, including the screens and electronics of smartphones, batteries of electric cars, satellites, and lasers,” said Joseph Cotruvo Jr., assistant professor and Louis Martarano Career Development Professor of Chemistry at Penn State and senior author of the study. “These elements are called rare earths, and they include chemical elements of atomic weight 57 to 71 on the periodic table. Rare earths are challenging and expensive to extract from the environment or from industrial samples, like waste water from mines or coal waste products. We developed a protein-based sensor that can detect tiny amounts of lanthanides in a sample, letting us know if it’s worth investing resources to extract these important metals.”
The research team reengineered a fluorescent sensor used to detect calcium, substituting the part of the sensor that binds to calcium with a protein they recently discovered that is several million times better at binding to lanthanides than other metals. The protein undergoes a shape change when it binds to lanthanides, which is key for the sensor’s fluorescence to “turn on.”
“The gold standard for detecting each element that is present in a sample is a mass spectrometry technique called ICP-MS,” said Cotruvo. “This technique is very sensitive, but it requires specialized instrumentation that most labs don’t have, and it’s not cheap. The protein-based sensor that we developed allows us to detect the total amount of lanthanides in a sample. It doesn’t identify each individual element, but it can be done rapidly and inexpensively at the location of sampling.”
The research team also used the sensor to investigate the biology of a type of bacteria that uses lanthanides — the bacteria from which the lanthanide-binding protein was originally discovered. Earlier studies had detected lanthanides in the bacteria’s periplasm — a space between membranes near the outside of the cell — but, using the sensor, the team also detected lanthanides in the bacterium’s cytosol — the fluid that fills the cell.
“We found that the lightest of the lanthanides — lanthanum through neodymium on the periodic table — get into the cytosol, but the heavier ones don’t,” said Cotruvo. “We’re still trying to understand exactly how and why that is, but this tells us that there are proteins in the cytosol that handle lanthanides, which we didn’t know before. Understanding what is behind this high uptake selectivity could also be useful in developing new methods to separate one lanthanide from another, which is currently a very difficult problem.”
The team also determined that the bacteria takes in lanthanides much like many bacteria take in iron; they secrete small molecules that tightly bind to the metal, and the entire complex is taken into the cell. This reveals that there are small molecules that likely bind to lanthanides even more tightly than the highly selective sensor.
“We hope to further study these small molecules and any proteins in the cytosol, which could end up being better at binding to lanthanides than the protein we used in the sensor,” said Cotruvo. “Investigating how each of these bind and interact with lanthanides may give us inspiration for how to replicate these processes when collecting lanthanides for use in current technologies.”
In addition to Cotruvo, the research team includes Joseph Mattocks and Jackson Ho at Penn State. This work is funded by the Penn State Eberly College of Science Department of Chemistry and the Penn State Huck Institutes of the Life Sciences.