As COVID-19 continues to spread, bottlenecks in supplies and laboratory personnel have led to long waiting times for results in some areas. In a new study, University of Illinois, Urbana-Champaign researchers have demonstrated a prototype of a rapid COVID-19 molecular test and a simple-to-use, portable instrument for reading the results with a smartphone in 30 minutes, which could enable point-of-care diagnosis without needing to send samples to a lab.
“If such a device and test were available, we could test for COVID-19 at public events, auditoriums, large gatherings and potentially even at home for self-testing. The results could be sent back to the appropriate public health system for coordination,” said Rashid Bashir, a professor of bioengineering and the dean of the Grainger College of Engineering at Illinois. Bashir co-led the study with electrical and computer engineering professor Brian Cunningham and mechanical science and engineering professor Bill King.
Typical tests for SARS-CoV-2, the virus that causes COVID-19, take a sample from a patient with a long nasopharyngeal swab, put that swab into a substance called viral transport media, and send it to a lab for a multistep process of extracting, isolating and multiplying the telltale RNA inside the virus. This RNA multiplication process, called RT-PCR, requires several temperature fluctuation cycles, specialized equipment and trained personnel, Cunningham said.
As reported in the Proceedings of the National Academy of Sciences, the Illinois team used a simpler process to analyze the viral transport media, called LAMP, which bypasses the RNA extraction and purification steps.
“LAMP only needs one temperature – 65 C – so it is much easier to control,” said graduate student Anurup Ganguli, the first author of the study. “Also, LAMP works more robustly than PCR, especially when there are contaminants in the test sample. We can just briefly heat the sample, break open the virus and detect the genetic sequence that specifically identifies SARS-CoV-2.”
The researchers compared the LAMP assay with PCR, first using synthetic nasal fluid spiked with the virus and then with clinical samples. They found the results were in agreement with PCR results, and they documented the sensitivity and specificity of the LAMP test.
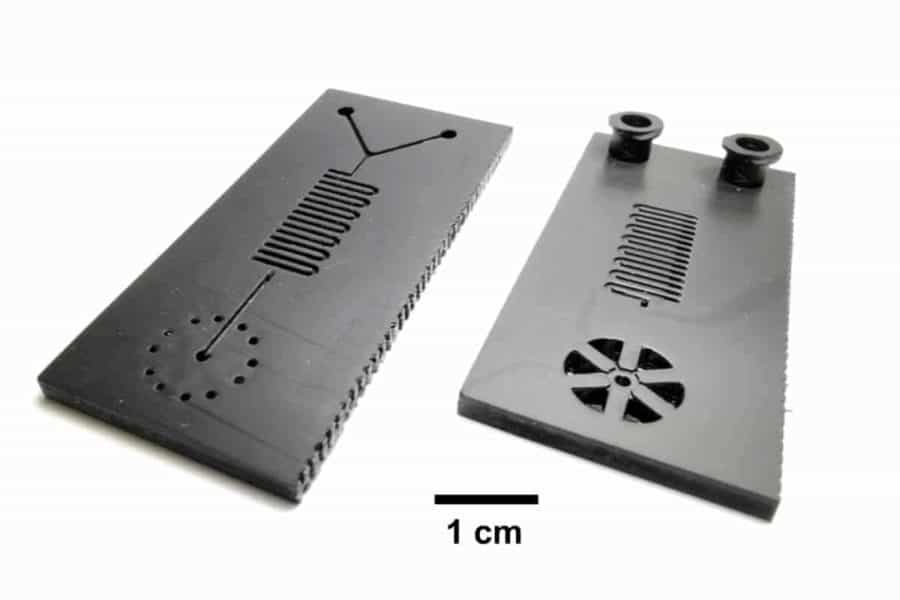
Then, the researchers incorporated the LAMP assay onto a small 3D-printed microfluidic cartridge that has two input slots for syringes: one for the sample-containing viral transport media, one for the LAMP chemicals. Once the two are injected, they react within the cartridge.
“We use modern, high speed additive manufacturing to make these cartridges. The entire thing can be quickly scaled up to hundreds of thousands of tests,” King said. “Production scale-up is typically the biggest obstacle for commercial applications of microfluidic cartridges, and we can overcome that obstacle using this new approach. Modern additive manufacturing is elastic and scalable, and it can be ramped up very quickly compared with legacy manufacturing technologies.”
The team is working with Fast Radius Inc., a Chicago-based technology company King co-founded, to manufacture the microfluidic cartridges.
The cartridge can be inserted into a hand-held portable instrument with a heating chamber, which heats the cartridge to 65 degrees Celsius for the duration of the reaction, and a smartphone cradle for reading the results. In approximately 30 minutes, a positive result will emit fluorescent light.
“The reader illuminates the liquid compartments with light from blue LEDs, while the phone’s rear-facing camera records a movie of the green fluorescent light being generated,” Cunningham said.
See a video of the cartridge, hand-held device and process on YouTube.
The researchers demonstrated the portable device with additional clinical samples, and found the results matched those of the standard PCR lab procedure.
The researchers are exploring whether the assay would work with saliva samples to eliminate the need for nasopharyngeal swabs, and collecting more patient data as they consider next steps for regulatory approvals, Bashir said.
The National Science Foundation, the National Institutes of Health and the Defense Advanced Research Projects Agency supported this work. Clinical samples were obtained from OSF HealthCare in collaboration with Dr. Sarah Stewart deRamirez and with support from the Jump Applied Research in Community Health through Engineering and Simulation partnership between OSF HealthCare and the U. of I.
Bashir, Cunningham and King are affiliated with the Beckman Institute for Advanced Science and Technology, the Carle Illinois College of Medicine and the Holonyak Micro and Nanotechnology Lab at Illinois. Bashir and Cunningham also are affiliated with the Cancer Center at Illinois and the Carl R. Woese Institute for Genomic Biology.
Editor’s notes: To reach Rashid Bashir, email [email protected]. To reach Brian Cunningham, email [email protected]. To reach Bill King, email [email protected].
The paper “Rapid isothermal amplification and portable detection system for SARS-CoV-2” is available online.