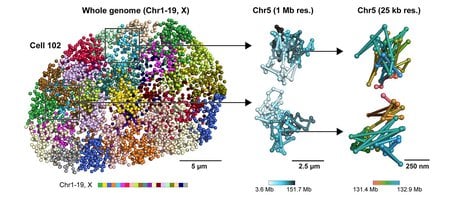
Nestled deep in the nucleus of each of your cells is what seems like a magic trick: Six feet of DNA is packaged into a tiny space 50 times smaller than the width of a human hair. Like a long, thin string of genetic spaghetti, this DNA blueprint for your whole body is folded and compacted into structures called chromosomes in order to fit within this space.
Also packed into the nucleus are structures called nuclear bodies, which are proteins that act like cellular machinery. And as if DNA and nuclear bodies were not enough to fit into the volume of a cubic micrometer, strands of RNA (that will be translated to proteins) are also crammed in throughout the nucleus.
The three-dimensional, spatial organization of the nucleus is important; it varies between individual cells and can contribute to differences in cellular states, for example, the phenotype of a brain cell versus a muscle cell.
Now, Caltech researchers have developed a new technique to image the nucleus, including its DNA, RNA, and proteins. This new technology, dubbed seqFISH+, has enabled the team to make multiple new discoveries about how the organization of the nucleus influences cellular function.
A paper about the research, conducted in the laboratory of Long Cai, professor of biology and biological engineering, appears in the journal Nature on January 27, 2021. Cai is an affiliated faculty member with the Tianqiao and Chrissy Chen Institute for Neuroscience at Caltech.
Other recently developed technologies can determine which genes are active in a cell and at what levels, but the advantage of seqFISH+ is that it can actually see the intact nuclear structure at high resolution. While previous imaging technologies could illustrate chromosome organization, they could not simultaneously image DNA, RNA, and proteins at a large scale. seqFISH+ integrates the strengths of these existing technologies to give a full picture of what is happening in a cell’s nucleus.
“The structure of a cell’s chromosomes and the way its DNA is folded have impacts on gene expression and regulation,” Cai explains. “Many chromosome studies are done by averaging out many cells, but gene expression varies between different cells. We really needed a way to see the structure within single cells.”
“There are many possible applications of seqFISH+. For example, it can be used to answer questions such as whether the nucleus of a cancer cell looks the same as that of a healthy cell,” Cai says. “We can use seqFISH+ to image cellular nuclei in intact tissues without having to break them up into individual cells.”
Indeed, as described in the Nature paper, seqFISH+ has already provided insights into nuclear structure. The team, led by graduate student Yodai Takei, presents several new major findings.
First, the team discovered that certain segments of the genome are positioned on the surface of nuclear body structures. The study suggests that these regions are not random; spatial proximity with nuclear bodies occurs in a deterministic way, meaning that nuclear bodies could act as “scaffolds” of the genome. Second, the team found that these interfaces between DNA and nuclear bodies agreed with previous findings about DNA-protein interactions that had been discovered with ChIP-seq, a technology that was developed in the Caltech laboratory of Barbara Wold (PhD ’78), Bren Professor of Molecular Biology.
Finally, the team reported that the nuclear state is very heterogeneous between different cells—more so than was previously realized—suggesting directions for future studies.
The paper is titled “Integrated spatial genomics reveals global architecture of single nuclei.” Takei is the study’s first author. Additional Caltech co-authors are research technician Jina Yun, former postdoctoral scholar Noah Ollikainen, research technician assistant Nico Pierson, graduate student Jonathan White, former postdoctoral scholar Sheel Shah (PhD ’17), Julian Thomassie (BS ’18), graduate student Chee-Huat Linus Eng, Professor of Biology and Heritage Medical Research Institute Investigator Mitchell Guttman, and Long Cai. Other co-authors are Shiwei Zheng, Shengbao Suo, and Guo-Cheng Yuan of the Dana-Farber Cancer Institute and the Harvard T.H. Chan School of Public Health. Funding was provided by the National Institutes of Health and the Paul G. Allen Frontiers Foundation Discovery Center.