The “invisible enemy” has not yet been disarmed. All specialists agree that SARS-CoV-2 will continue to circulate in the same way as the influenza or cold viruses. The search for medications against Covid-19 remains as important as it was in the early days of 2020, for several reasons. First of all, the virus will evolve over time. The scientists have no doubt that the appearance of new variants will challenge the vaccine shield. Secondly, some groups in the population, such as those whose immune system is depressed, are not really protected by the inoculation. Not to mention those who refuse to be vaccinated, and who “will need to be taken care of”, points out Jean Dubuisson, CNRS senior researcher at the Centre for Infection and Immunity of Lille (CIIL),1 in northern France.
Three main strategies are available to the laboratories that seek to provide physicians with new therapeutic solutions. Repurposing is the most rapid route. This involves searching among existing molecules in the pharmacopoeia to find those that might exert antiviral activity. One of the advantages of this approach is that much of their development has already been completed. The second option involves the search for new and specific antiviral compounds, which is a lengthy process strewn with pitfalls. Indeed, an average of ten years is necessary to take a drug to market, involving an investment that can exceed €1 billion. And finally, monoclonal antibodies could also become weapons of choice against SARS-CoV-2.
Here are a few examples of the advances achieved in these three research areas, taken from the numerous projects underway in CNRS laboratories.
Repurposing: exploring databanks of molecules
During the early weeks of the pandemic, laboratories throughout the world were searching the pharmaceutical arsenal to find a molecule that might be effective against Covid-19. One of them was the VirPath team at the CIRI2 in Lyon (southeastern France). Its members had previously determined that diltiazem, a drug prescribed to treat hypertension, was effective against the influenza virus. Boosted by this finding, the scientists tried to find out whether it would also have an antiviral effect on SARS-CoV-2. To achieve this, they first performed in vitro tests, followed by experiments using a reconstituted human epithelial cell model.
This model is made up of primary cells and displays characteristics very similar to those observed in the human respiratory tract. It is “particularly well suited to evaluating candidate molecules against respiratory viruses, and enables the identification of the most effective compounds”, explains Olivier Terrier, scientist at the CIRI and winner of the CNRS 2021 Bronze Medal, who in collaboration with the team led by Roger Legrand at the Infectious Diseases Models for Innovative Therapies (IDMIT) laboratory, also carried out assessments in animals.
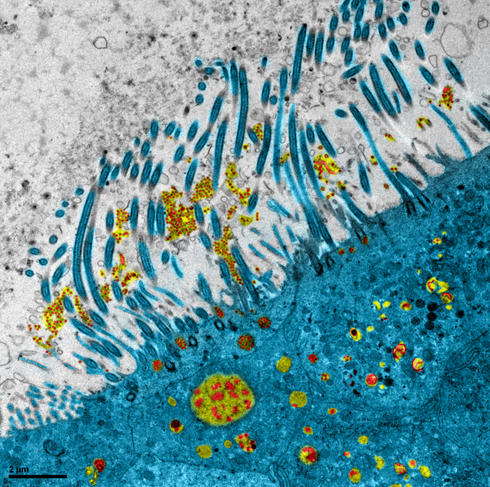
The scientists showed that diltiazem does not directly target the virus but induces a specific part of the interferon response. This immune reaction is the first line of defence against pathogens. Terrier is hoping that “this drug may be of interest alone or in combination with classic antivirals that target the infection”. Thanks to its mode of action, it could be included in therapies against a broader panel of respiratory viruses. A phase 2 clinical trial on the treatment of severe forms of influenza has just been completed; the scientists are now awaiting the green light from the health authorities before initiating tests on Covid-19, in collaboration with the start-up company, Signia Therapeutics.
Another lead comes from scientists at the CIRI and the Paris-based Saint-Antoine Research Centre, who have discovered that naproxen, a widely-used anti-inflammatory and analgesic, also exerts an antiviral effect against SARS-CoV-2. The molecule seems to interact with the N protein in the virus and thus disturb its replication. Thanks to Assistance Publique–Hôpitaux de Paris (AP-HP), a clinical trial called Enacovid is currently underway in patients with Covid-19.
High-throughput screening
The CIIL has also discovered another promising molecule. Taking advantage of their automated high-security platform, its scientists tested 1942 compounds from a large chemical library for their antiviral activity on cell cultures. The result of this high-throughput screening programme was that some sixty active ingredients appeared to block the spread of the virus. “Our experience taught us that with this type of screening, we would find several dozen antiviral molecules, but not all of them could be used,” explains Dubuisson. “For example, some antidepressants have antiviral activity, but we know that they are not suitable for use in this context, since they may affect other functions.”
Following a search of the bibliography and a further series of tests, the scientists were able to reduce the list of candidates to around ten, and ultimately focused on a single compound, clofoctol, which is used to treat bacterial infections of the respiratory tract.
Having demonstrated in vitro that clofoctol blocked viral replication, the team then performed experiments on mice susceptible to the coronavirus and the results proved very encouraging. In vivo, the drug was still effective against viral replication and reduced lung degradation in the rodents. Furthermore, the tests showed that the molecule also displayed an anti-inflammatory effect. This is important because the inflammation induced by SARS-CoV-2 worsens the health status of patients. CIIL scientists have just initiated a clinical trial on the repurposing of clofoctol, which will be performed on a panel of 684 patients.
New molecules for new targets
As early as 2003, when the SARS epidemic caused around 800 deaths worldwide, scientists at the AFMB3 laboratory in Marseille (southeastern France) had understood that coronaviruses would eventually return and cause harm. This prompted them, in subsequent years, to study the proteins in SARS-CoV-1. They were particularly interested in its replication complex, the enzymes that enable the virus to reproduce once it has entered a host cell. Indeed, many antivirals are effective because they inactivate this complex. After much effort, the researchers succeeded in extracting this machinery and making it function in vitro. “This work would have made it possible to screen for antiviral molecules as far back as 2012,” says Bruno Canard, CNRS senior researcher at the AFMB. However, ten years ago, it was difficult to find funding for the high-throughput screening of emerging viruses, and the scientific community moved on to other topics.
The 2020 pandemic caused them to resume their work. They immediately noted that the replication complex of SARS-CoV-2 was almost identical to that of its predecessor, and were thus rapidly in a position to initiate the search for a molecule capable of blocking its operation. “Thanks to our screening platform, managed by Jean-Claude Guillemot, we managed to test about 20,000 compounds from the national chemical library,” explains Canard. As a result, the team identified some ten candidate molecules that could be developed as treatments against Covid-19. These are very preliminary results, but the AFMB now benefits from a significant advantage: it belongs to the European IMI-Care project, a public-private partnership born during the pandemic, which involves more than thirty partners and scientific institutions (including the CNRS) from eight countries, alongside major pharmaceutical companies and start-ups.
This exchange of skills sped up the study of these molecules. The AFMB can devote itself to screening new active ingredients and studying their modes of action, while other laboratories have taken charge of their optimisation and various preclinical tests. The AFMB is also working on AT-527, an antiviral recently discovered by the Boston-based US firm ATEA. Due to their previous work on the polymerase of SARS-CoV-1, ATEA and the AFMB established a partnership to determine the mechanism of action of this very promising agent. Clinical trials then demonstrated its efficacy in patients, which justified its entry into phase 3 in June 2021 in the US. The same drug is also being developed to treat hepatitis C and the dengue virus, which has long been a focus of the AFMB.
Broad-spectrum antivirals
Before the pandemic, scientists at the IRIM4 were investigating a novel area; they were searching for a molecule that would be effective against a broad panel of RNA viruses. To achieve this, it was necessary to target not just the pathogens themselves but the cellular proteins they hijacked to their benefit in order to replicate. The researchers thus identified a helicase called DHX9. This protein, whose function is to unwind RNA molecules, is essential for the replication of HIV, chikungunya, Ebola, influenza and dengue, which made it a target of choice.
“We used bioinformatic tools for the in silico identification of agents that might be able to inactivate helicase DHX9,” explains Laurent Chaloin, CNRS senior researcher at the IRIM. The team then tested the antiviral effects of several candidate compounds experimentally before focusing on the most promising, which in cell cultures did prevent replication of the RNA viruses mentioned above. It was at that time that the Covid-19 pandemic started, and of course, the scientists tested the molecule on SARS-CoV-2. They found that it displayed potent antiviral action on this RNA pathogen as well. Indeed, as they were able to demonstrate, the coronavirus also uses DHX9 to its benefit.
As well as its astonishing spectrum of activity, this molecule has the advantage of a very low toxicity. “Our tests confirmed that blocking helicase DHX9 had no harmful effects on human cells,” confirms Jean-Marie Péloponèse, also a CNRS scientist at the IRIM. It appears that, to fulfil their own needs, the cells contain other helicases that are not affected by this compound.
Lengthy efforts to optimise the molecule and many series of tests now await the scientists. In the short term, they hope to perform an in vivo study on mice susceptible to SARS-CoV-2 before the end of the year. “We hope to take this work to the clinical phase. After that, we’ll need to find partners – whether pharmaceutical start-ups or firms – to go any further,” concludes Péloponèse.
Immunotherapies: a new battlefront against the virus
Antibodies are one of the most powerful weapons in our immune system. Produced by B- lymphocytes in response to an attack, they recognise and neutralise pathogenic agents in a specific manner. They have been used since the 1980s to treat a variety of conditions, including numerous cancers, Crohn’s disease and psoriasis. From the start of the pandemic, scientists and pharmaceutical companies considered their use against SARS-CoV-2. Thus in October 2020, Donald Trump received an experimental treatment based on monoclonal antibodies that has since been adopted by several countries, including France.
The first step in developing an antibody-based treatment is to choose the correct target to be inactivated. Unsurprisingly, most research is focusing on the Spike (S) protein of the virus, which gives it its chestnut shell appearance and allows it to attach to and infect the cells of its host.
However, inactivating Spike can have some unexpected effects. This was shown by a French-Singaporean team, in which the VirPath group is involved. During their experiments, they screened in vitro a bank of antibodies targeting the domain of the Spike protein that enables it to attach itself to cells. Some of these antibodies were very effective, but others, while attaching equally well to Spike, also stimulated membrane fusion and the formation of syncytia, fused cells which therefore contain several nuclei. This fusion property may constitute a problem because syncytia can exacerbate the chronic tissue damage observed in patients. “The therapeutic use of antibodies has enormous potential, but our work has underlined the importance of upstream preclinical characterisation in order to avoid potential adverse effects,” warns Terrier.
Soothing the immune system
At Institut Cochin,5 in Paris, scientists have been concentrating on a different target. Their idea is not to attack the virus with monoclonal antibodies but to modulate the immune response of patients. Indeed, in many serious cases of Covid-19, it is the disproportionate reaction of the body’s defences that compromises survival. This is due to a category of killer cells in the immune system called MAIT cells. Activated by cytokines, molecules produced (amongst others) by macrophages, MAIT accumulate in the lungs and can kill both infected and healthy cells. In many sufferers, they are associated with degradation of the pulmonary epithelium and respiratory distress. For this reason, at Hôpital Bichat (also in Paris), the degree of activation of MAIT cells already serves as a prognostic marker; if this is high, then the state of the patient is likely to deteriorate shortly.
Monoclonal antibodies may help to limit this over-reaction by the immune system. “We have identified two monoclonal antibodies that are able to inactivate MAIT,” announces Agnès Lehuen, CNRS senior researcher at Institut Cochin. “The first is already marketed by GSK to treat certain severe cases of Crohn’s disease, while the second is still experimental and has only been the subject of preclinical studies so far.” Still, the scientists have shown that the latter clearly inactivated MAIT cells during in vitro experiments. The next stage consists in optimising and testing this monoclonal antibody so that clinical trials can be initiated.
Of all the antibodies and molecules being studied in different laboratories to treat Covid-19, it is impossible to say which will one day be administered to patients. There are many candidates and very few will be chosen. However, the importance of this work goes beyond the development of therapeutic strategies. “We learn an awful lot about viruses every time we discover a new antiviral molecule,” explains Canard. “We obtain information on their structure and their interactions with the human body.” These efforts also enable the use of new tools in the search for treatments. These tools and the knowledge they generate will be crucial when the next pandemic breaks out somewhere in the world, hopefully as late as possible.

