Researchers studying how cancer spreads into bone have made a surprising discovery that suggests several investigational anti-cancer therapies just entering the drug-development pipeline may not have the desired effect.
The study in The Journal of Experimental Medicine indicates that the drugs inadvertently may promote cancer.
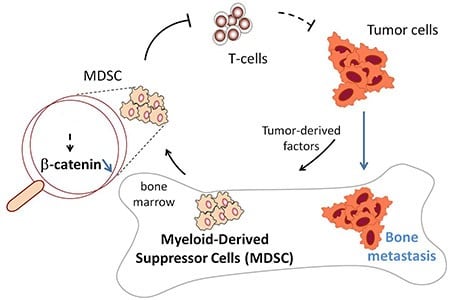
The researchers fromWashington University School of Medicine in St. Louis were focusing on cells that can be found in great numbers in several types of cancer. These cells, called myeloid-derived suppressor cells (MDSCs) interfere with T-cells from the immune system, making it difficult for T-cells to attack and kill tumor cells.
The therapies being developed target a signaling protein expressed in high amounts in some cancer cells. The protein, beta-catenin, is thought to promote tumor growth, but the investigators found that shutting it down in myeloid cells in bone marrow may have the unintended consequence of increasing the number of MDSCs and making it harder for the body’s immune system to attack growing tumors.
“The idea is that cancer cells often have elevated beta-catenin signaling, leading to more tumor cell proliferation, so many scientists have hypothesized that drugs that lower beta-catenin levels could fight cancer,” said senior investigator Roberta Faccio, PhD. “But we think that such a strategy actually could contribute to the proliferation of tumors. Our findings suggest that although some inhibitors of beta-catenin may interfere with tumor cell growth, they also could expand the population of the MDSCs and contribute to the development and spread of cancer.”
Faccio, an associate professor of orthopaedic surgery whose laboratory studies how cancer spreads into bone, worked with first author Aude-Hélène Capietto, PhD, to learn exactly how MDSCs inhibit the immune system’s ability to ward off cancer. In a series of experiments, they analyzed genetically engineered mice to learn how those cells contribute to the proliferation and spread of tumor cells.
As expected, they found that mice with tumors had more myeloid-derived suppressor cells, elevating the likelihood that their tumors would grow and spread. Surprisingly, the MDSCs in mice with tumors also had lower amounts of beta-catenin. That’s important because some drugs being considered as potential cancer treatments block beta-catenin. This study’s findings suggest that could be harmful.
MDSCs are immature immune cells that are supposed to “grow up” to become specialized immune cells. But in the presence of tumors, the cells don’t develop properly. Instead, they suppress the immune system’s T-cells, making it easier for tumors to grow and spread.
That’s what occurred in the genetically engineered mice. They had lower levels of beta-catenin in MDSCs, leading to more such cells as well as more aggressive tumors.
But the process worked in reverse, too. When the researchers raised beta-catenin levels in the MDSCs of mice with cancer, the number of their myeloid-derived suppressor cells declined, and their tumor growth slowed.
“A lot of cancer treatments don’t work very well, and they kill both cancer cells and healthy cells,” said Capietto. “But our findings indicate that myeloid-derived suppressor cells may be good targets for therapy because we know they induce tumor growth.”
Faccio believes the pathway involving beta-catenin behaves the same way in people as it does in mice because they got the same results in studies of MDSCs from cancer patients.
“When we isolated cells from patients with pancreatic cancer, we found the very same things we had observed in mice with cancer,” Faccio said. “So it was a confirmation that this pathway is quite relevant in the progression of cancer.”
In addition to being published online, the findings also were presented this fall at the annual meeting of the American Society for Bone and Mineral Research, where Capietto, a post-doctoral research associate in Faccio’s laboratory, received the society’s President’s Award for the highest-ranking abstract submitted by a student.