In addition to being a transportation fuel, methanol is an important ingredient in plastics, adhesives and solvents.
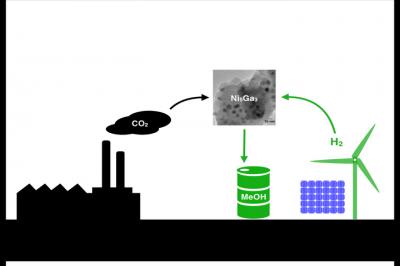
Now, researchers say they’ve found a clean and cheap way to convert the greenhouse gas carbon dioxide into methanol.
Scientists from the Technical University of Denmark, Stanford University and SLAC National Accelerator Laboratory found a new nickel-gallium catalyst that turns hydrogen and carbon dioxide into methanol with less side-products than the traditional catalyst.
“Methanol is processed in huge factories at very high pressures using hydrogen, carbon dioxide and carbon monoxide from natural gas,” said SLAC scientist Felix Studt. “We are looking for materials than can make methanol from clean sources under low-pressure conditions, while generating low amounts of carbon monoxide.”
Added Jens Nørskov, a chemical engineer at Stanford: “Imagine if you could synthesize methanol using hydrogen from renewable sources, such as water split by sunlight, and carbon dioxide captured from power plants and other industrial smokestacks…. Eventually we would also like to make higher alcohols, such as ethanol and propanol, which, unlike methanol, can be directly added to gasoline today.”
Industrial methanol
In a normal methanol plant, natural gas and water are turned into so-called synthesis gas, or syngas, which is mostly carbon monoxide, carbon dioxide and hydrogen. That, in turn, is turned into methanol via high-pressure and a catalyst made of copper, zinc and aluminum.
“We spent a lot of time studying methanol synthesis and the industrial process,” said Studt. “It took us about three years to figure out how the process works and to identify the active sites on the copper-zinc-aluminum catalyst that synthesize methanol.”
Once the team understood the molecular workings of methanol synthesis, they looked for a new catalyst to synthesize methanol at low pressures with just hydrogen and carbon dioxide. To help the hunt, they searched for promising candidates in a large database developed at SLAC.
“The technique is known as computational materials design,” said Nørskov. “You get ideas for new functional materials based entirely on computer calculations. There is no trial-and-error in the lab first. You use your insight and enormous computer power to identify new and interesting materials, which can then be tested experimentally.”
The researchers compared the copper-zinc-aluminum catalyst with thousands of other materials in the database. The most promising candidate turned out to be a little-known compound called nickel-gallium. “Once we got the name of the compound out of the computer, someone still had to test it,” Nørskov said. “We don’t do lab experiments here, so we have to have a good experimental partner.”
Lab tests confirmed it was the right choice. Nickel-gallium made more methanol than the conventional copper-zinc-aluminum catalyst, and less of the carbon monoxide byproduct. “You want to make methanol, not carbon monoxide,” Chorkendorff said. “You also want a catalyst that’s stable and doesn’t decompose. The lab tests showed that nickel-gallium is, in fact, a very stable solid.” Nickel is fairly abundant, and gallium is already is widely used in the electronics industry. Because of this, the new catalyst could eventually be scaled up for industrial use, the authors say.