Ketamine, a stalwart in the medical arsenal, serves many roles – from sedative to painkiller to anesthetic to antidepressant. Its effects on the brain are well-documented, yet the precise link between its molecular target and the sweeping changes it induces in neural activity has remained elusive.
Now, a collaborative effort by scientists across four Boston institutions has yielded a computational model that bridges this gap, potentially empowering physicians to wield ketamine with greater finesse.
The research team, spanning MIT, Boston University, Massachusetts General Hospital, and Harvard University, published their findings on May 20 in the Proceedings of the National Academy of Sciences. By incorporating previously overlooked physiological nuances, their model offers predictions that could help optimize the use of this versatile drug.
“When physicians understand what’s mechanistically happening when they administer a drug, they can possibly leverage that mechanism and manipulate it,” said study lead author Elie Adam, a Research Scientist at MIT poised to join the Harvard Medical School faculty and launch a lab at MGH. “They gain a sense of how to enhance the good effects of the drug and how to mitigate the bad ones.”
Deciphering the Dance of Ions and Voltages
At the heart of the study lies a meticulous simulation of ketamine’s blockade of NMDA receptors in the brain’s cortex. These receptors, when open, allow ions to flow in and out of neurons, modulating their electrical properties. Ketamine, the model shows, is a particularly potent blocker, impeding this flow and altering the delicate dance of neural activity.
The model goes beyond its predecessors by capturing the intricate interplay between the voltage across a neuron’s membrane, its spiking behavior, and the blocking and unblocking of NMDA receptors. This dynamic, the researchers assert, imbues NMDA receptors with a swifter responsiveness than their slow closing speed might suggest.
“Physiological details that are usually ignored can sometimes be central to understanding cognitive phenomena,” said co-corresponding author Nancy Kopell, a professor of math at BU. “The dynamics of NMDA receptors have more impact on network dynamics than has previously been appreciated.”
From Molecular Mechanics to Brain Waves
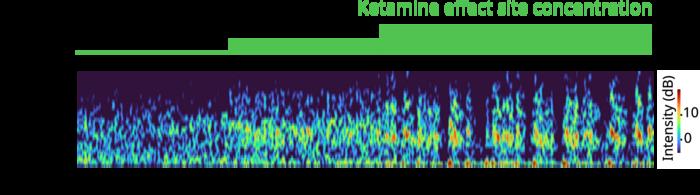
Armed with their model, the scientists simulated how varying doses of ketamine would alter the activity of a model brain network, successfully recapitulating the brain waves and neural spiking patterns observed in human and animal studies. Low doses amplified fast gamma frequency waves, while higher, consciousness-suppressing doses periodically disrupted these waves with slow delta wave “down” states.
The simulations revealed several key mechanisms underlying these phenomena. Notably, ketamine can disinhibit network activity by quelling certain inhibitory interneurons, and the bursts of spiking it induces can synchronize into the observed gamma waves. The model also suggests that at higher doses, the impaired NMDA-receptor kinetics can’t sustain the excitatory neurons’ gamma-frequency activity, leading to the periodic down states.
Intriguingly, the model hints at a potential mechanism for ketamine’s antidepressant effects. It posits that the drug’s amplification of gamma activity could entrain neurons expressing a beneficial, inflammation-reducing peptide called VIP. While speculative, this connection points to therapeutic aspects of ketamine that may extend beyond its antidepressant properties.
“The understanding that the sub cellular details of the NMDA receptor can lead to increased gamma oscillations was the basis for a new theory about how ketamine may work for treating depression,” Kopell said.
As physicians continue to explore the multifaceted potential of ketamine, this computational model offers a roadmap to navigate its complex effects on the brain. By illuminating the intricate dance of molecules and networks, it paves the way for more targeted, effective use of this essential medicine.