Millions of people with paralysis reside in the United States. Sometimes their paralysis comes gradually, as occurs in ALS. Sometimes it arrives suddenly, as in Degray’s case.
Now 64, Degray became quadriplegic on Oct. 10, 2007, when he fell and sustained a life-changing spinal-cord injury. “I was taking out the trash in the rain,” he said. Holding the garbage in one hand and the recycling in the other, he slipped on the grass and landed on his chin. The impact spared his brain but severely injured his spine, cutting off all communication between his brain and musculature from the head down.
“I’ve got nothing going on below the collarbones,” he said.
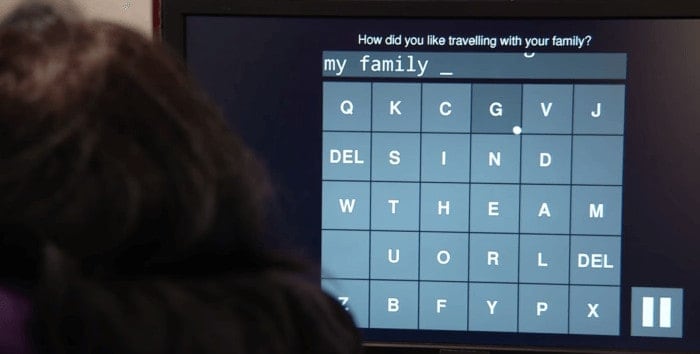
Degray received two device implants at Henderson’s hands in August 2016. In several ensuing research sessions, he and the other two study participants, who underwent similar surgeries, were encouraged to attempt or visualize patterns of desired arm, hand and finger movements. Resulting neural signals from the motor cortex were electronically extracted by the embedded recording devices, transmitted to a computer and translated by Shenoy’s algorithms into commands directing a cursor on an onscreen keyboard to participant-specified characters.
The researchers gauged the speeds at which the patients were able to correctly copy phrases and sentences — for example, “The quick brown fox jumped over the lazy dog.” Average rates were 7.8 words per minute for Degray and 6.3 and 2.7 words per minute, respectively, for the other two participants.
A tiny silicon chip
The investigational system used in the study, an intracortical brain-computer interface called the BrainGate Neural Interface System*, represents the newest generation of BCIs. Previous generations picked up signals first via electrical leads placed on the scalp, then by being surgically positioned at the brain’s surface beneath the skull.
An intracortical BCI uses a tiny silicon chip, just over one-sixth of an inch square, from which protrude 100 electrodes that penetrate the brain to about the thickness of a quarter and tap into the electrical activity of individual nerve cells in the motor cortex.
This is like one of the coolest video games I’ve ever gotten to play with.
Henderson likened the resulting improved resolution of neural sensing, compared with that of older-generation BCIs, to that of handing out applause meters to individual members of a studio audience rather than just stationing them on the ceiling, “so you can tell just how hard and how fast each person in the audience is clapping.”
Shenoy said the day will come — closer to five than 10 years from now, he predicted —when a self-calibrating, fully implanted wireless system can be used without caregiver assistance, has no cosmetic impact and can be used around the clock.
“I don’t see any insurmountable challenges.” he said. “We know the steps we have to take to get there.”
Degray, who continues to participate actively in the research, knew how to type before his accident but was no expert at it. He described his newly revealed prowess in the language of a video game aficionado.
“This is like one of the coolest video games I’ve ever gotten to play with,” he said. “And I don’t even have to put a quarter in it.”
The study’s results are the culmination of a long-running collaboration between Henderson and Shenoy and a multi-institutional consortium called BrainGate. Leigh Hochberg, MD, PhD, a neurologist and neuroscientist at Massachusetts General Hospital, Brown University and the VA Rehabilitation Research and Development Center for Neurorestoration and Neurotechnology in Providence, Rhode Island, directs the pilot clinical trial of the BrainGate system and is a study co-author.
“This incredible collaboration continues to break new ground in developing powerful, intuitive, flexible neural interfaces that we all hope will one day restore communication, mobility and independence for people with neurologic disease or injury,” said Hochberg.
Stanford research assistant Christine Blabe was also a study co-author, as were BrainGate researchers from Massachusetts General Hospital and Case Western University.
The study was funded by the National Institutes of Health (grants R01DC014034, R011NS066311, R01DC009899, N01HD53404 and N01HD10018), the Stanford Office of Postdoctoral Affairs, the Craig H. Neilsen Foundation, the Stanford Medical Scientist Training Program, Stanford BioX-NeuroVentures, the Stanford Institute for Neuro-Innovation and Translational Neuroscience, the Stanford Neuroscience Institute, Larry and Pamela Garlick, Samuel and Betsy Reeves, the Howard Hughes Medical Institute, the U.S. Department of Veterans Affairs, the MGH-Dean Institute for Integrated Research on Atrial Fibrillation and Stroke and Massachusetts General Hospital.
Stanford’s Office of Technology Licensing holds intellectual property on the intercortical BCI-related engineering advances made in Shenoy’s lab.
Stanford’s departments of Neurosurgery and of Electrical Engineering also supported the work. Shenoy and Henderson are members of Bio-X and the Stanford Neuroscience Institute.
*CAUTION: Investigational Device. Limited by Federal Law to Investigational Use.