Rutgers scientists have developed catalysts that can convert carbon dioxide – the main cause of global warming – into plastics, fabrics, resins and other products.
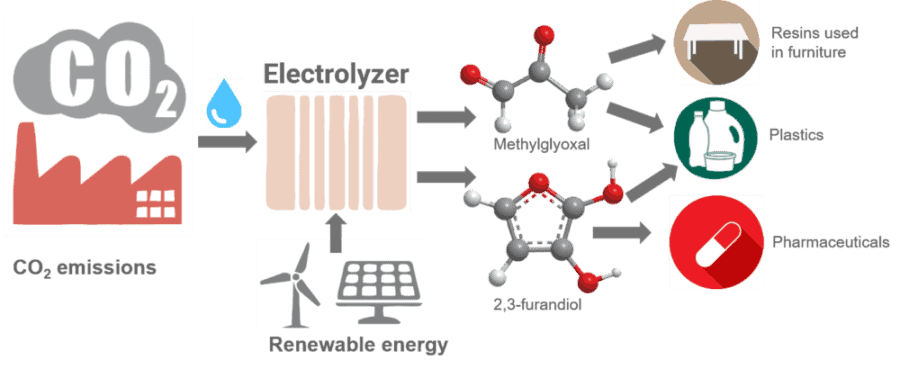
The electrocatalysts are the first materials, aside from enzymes, that can turn carbon dioxide and water into carbon building blocks containing one, two, three or four carbon atoms with more than 99 percent efficiency. Two of the products created by the researchers – methylglyoxal (C3) and 2,3-furandiol (C4) – can be used as precursors for plastics, adhesives and pharmaceuticals. Toxic formaldehyde could be replaced by methylglyoxal, which is safer.
The discovery, based on the chemistry of artificial photosynthesis, is detailed in the journal Energy & Environmental Science.
“Our breakthrough could lead to the conversion of carbon dioxide into valuable products and raw materials in the chemical and pharmaceutical industries,” said study senior author Charles Dismukes, Distinguished Professor in the Department of Chemistry and Chemical Biology and Department of Biochemistry and Microbiology at Rutgers University–New Brunswick. He is also a principal investigator at Rutgers’ Waksman Institute of Microbiology.
Previously, scientists showed that carbon dioxide can be electrochemically converted into methanol, ethanol, methane and ethylene with relatively high yields. But such production is inefficient and too costly to be commercially feasible, according to study lead author Karin Calvinho, a chemistry doctoral student in Rutgers’ School of Graduate Studies.
However, carbon dioxide and water can be electrochemically converted into a wide array of carbon-based products, using five catalysts made of nickel and phosphorus, which are cheap and abundant, she said. The choice of catalyst and other conditions determine how many carbon atoms can be stitched together to make molecules or even generate longer polymers. In general, the longer the carbon chain, the more valuable the product.
Based on their research, the Rutgers scientists earned patents for the electrocatalysts and formed RenewCO₂, a start-up company. The next step is to learn more about the underlying chemical reaction, so it can be used to produce other valuable products such as diols, which are widely used in the polymer industry, or hydrocarbons that can be used as renewable fuels. The Rutgers experts are designing, building and testing electrolyzers for commercial use.



Carbon dioxide has nothing to do with non-existent “global warming” which even the bedwetting eco-terrorists don’t call it any more. There is no scientific explanation of how a gas that is 0.044% of the atmosphere could possibly raise temperatures. It cannot do that.
Based on the lack of sunspot activity recently, global cooling is what is happening and is a far greater worry. Cold kills, heat does not.
If you want to reduce atmospheric CO2 (which has greened the earth and increased crop yields) just plant more trees and bushes.
The value of this process should only be based on the polymer products, not the CO2 reduction.
Why?
The process is going to lead to a significant reduction in atmospheric CO2 only if the electricity comes from nuclear or renewable sources. Otherwise, the energy to reduce CO2 would require at least that much energy produced from burning (oxidizing) fossil fuels.
“energy to reduce CO2” in the last sentence, I mean chemical reduction of CO2 to carbon. In other places, I am referring to reducing the atmospheric content of CO2.