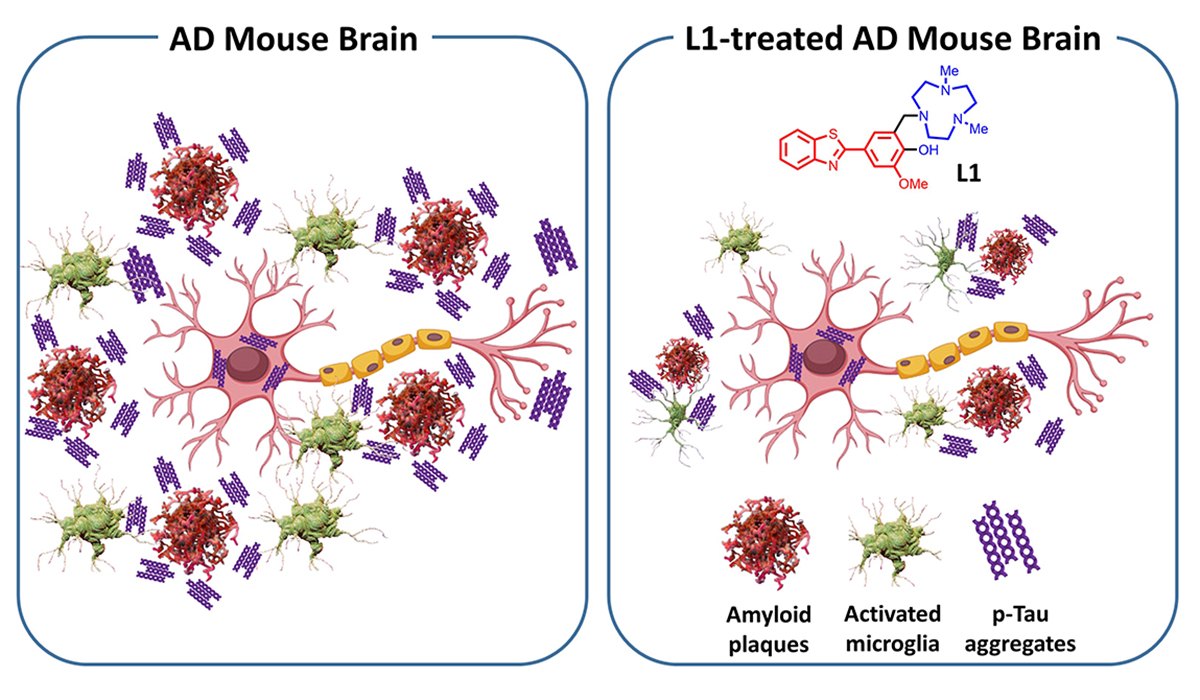
When tested in brain cells and in a mouse model of Alzheimer’s disease, a new compound significantly reduced the number of amyloid plaques in the brain, lessened brain inflammation and diminished other molecular markers of the disease.
The researchers who developed the compound report their findings in the journal ACS Chemical Neuroscience.
Recent studies show that aggregates of p-tau collect around amyloid plaques, increasing the neuroinflammation that is associated with Alzheimer’s, Mirica said.
“It’s possible that the amyloid plaques somehow trigger the aggregation of the p-tau proteins in the extracellular environment,” he said. “And this process then spurs the spread and formation of p-tau inside the neurons, where it starts to clump together.”
This clumping leads to the formation of “neurofibrillary tangles” in the neurons that disrupt cell function and ultimately lead to cell death, he said.
Scientists do not clearly understand the mechanisms that lead to Alzheimer’s disease. Many studies have focused on dissolving or disrupting the formation of the amyloid plaques, which are composed of a protein fragment called the beta-amyloid peptide.
“Everybody was trying to attack the amyloid plaques to dissolve them, but we’ve learned that Alzheimer’s is a bit more complicated than we thought,” Mirica said.
Previous research has found that soluble beta-amyloid fragments are more dangerous to brain health even before they aggregate into plaques.
“Studies have found strong evidence that these soluble peptides are the most neurotoxic species and are causing memory loss and neuron cell death,” Mirica said. Plaque formation might be an attempt by the brain to neutralize the threat, he said.
Metal ions such as copper and iron also play a role in Alzheimer’s pathology. These metals form associations with the beta-amyloid peptide, stabilizing the more dangerous, soluble beta-amyloid species. Metal ions also contribute to the damaging oxidative stress and brain inflammation seen in Alzheimer’s.
“To address these different pathological aspects of Alzheimer’s disease, we developed a compound known as L1 that interacts with different regions of the beta-amyloid peptide, as well as with metal ions,” Mirica said. “When tested in mice genetically predisposed to develop Alzheimer’s-like pathologies, our compound could cross the blood-brain barrier, reduce neuroinflammation and decrease the levels of amyloid plaques and of p-tau aggregates associated with these plaques.”
The findings suggest that drugs that interact with different regions of amyloid-beta protein fragments and other Alzheimer’s-associated peptides and metal ions could improve outcomes for people with Alzheimer’s disease.
The National Institutes of Health and Alzheimer’s Association supported this research.
Editor’s notes:
To reach Liviu Mirica, email [email protected].
The paper “A multifunctional chemical agent as an attenuator of amyloid burden and neuroinflammation in Alzheimer’s disease” is available online and from the U. of I. News Bureau.