Dye pigments are often toxic, so researchers around the world have long been looking for effective ways to make non-toxic, recyclable and sustainable colours instead. The answer lies in nanotechnology and nature’s own methods.
Dyes that humans make often contain substances that are harmful to the environment. But it doesn’t have to be that way anymore.
“We’re mimicking normal color mechanisms in nature,” says Jon Otto Fossum, a professor at the Norwegian University of Science and Technology’s (NTNU) Department of Physics.
He and others from his research group are collaborating with a research team at the University of Bayreuth in Germany. They have discovered a practical and inexpensive method for producing colours that are sustainable and non-toxic. These colors will be both recyclable and sustainable as well.
The researchers are using nanotechnology, where they manipulate materials all the way down to the atomic level.
Their work was first presented during postdoctoral fellow Paulo Michels-Brito’s PhD dissertation. The results have now been published in Science Advances.
Colors in nature are different
When your house or car has been given a color, it is usually paint, stain or varnish that has been applied. The color of the house or car depends entirely on the material of the paint or varnish. The colour we see is the part of the light spectrum that the material does not absorb.
But nature’s way of making colors is often completely different.
“Birds, fish, insects, some plants and berries, soap bubbles, the rainbow and so on have colors that are based on a partial reflection of light waves,” says Fossum.
What we see is light that is reflected in nanothin structures in the material. The light reflected in the different layers is amplified through what’s called “constructive interference.” This creates what we see as colors.
To imitate this way of making colors, researchers at NTNU’s Department of Physics have used nanothin layers from a seemingly humble material.
Clay.
Nanothin sheets of clay
“Most people don’t think of clay as transparent. But if we isolate nanothin sheets of clay or graphene, for example, they become almost completely transparent. They don’t reflect light and are colorless,” says Fossum.
But by combining two such transparent clay sheets and putting an additional substance in between, it is possible to bring out colors.
“We can make clay nanosheets less transparent by making and using a double nanothin layer with an element between them. In this example, we used the element cesium, but other substances can also be used,” says Fossum.
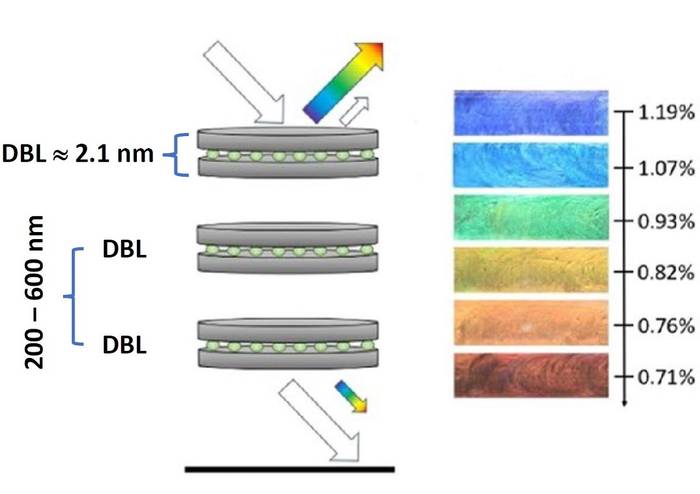
The researchers call the double nanothin clay layers DBL (“Double Layers”). Double clay layers are still partially transparent, but reflect some light that produces color.
Distance between layers determines color
“The mechanism we work with is called ‘structural color’ because it’s the nanostructure, and not the specific material, that gives rise to the colors,” says Fossum. “That’s why structural colors are also often called ‘physical colors,’ whereas traditional pigments are often called “chemical colors.”
The material has properties at the nano level that make it possible to reflect light from different depths in the structure. This can in turn result in different colors because the combined light from the different layers amplify each other in the same way as we see in soap bubbles or rainbows.
“The distance between the double clay layers is what produces the color we see. We can control the distance by means of the clay concentration or salt content in the material, such as water, that the clay is suspended in,” Fossum explains.
In this example, the water is the “matrix” of the clay layers. This matrix has to be absolutely transparent for the double clay layers to reflect and give rise to the colors.
Complex materials
“From a basic science perspective, this system is a textbook example of what we mean by complexity, including self-assembly of complex materials,” says Fossum.
The Nobel Prize in Physics for 2021 was given to scientists who research complex systems.
“A complex system is made up of several components that together contribute to the desired functionality, in this case colours,” says Fossum.
The colours themselves come from the clay layers, while the matrix keeps everything in place. The result is more than just the sum of the components, because they’re self-assembled in a special way at the nanoscale, Fossum says.
Environmentally friendly instead of toxic
All this could bring about a lot of changes and provide a far more environmentally friendly way of coloring many products in lots of areas.
“Chemical pigments are often toxic, whereas physical colors can be produced from anything that is almost transparent,” says Fossum.
This applies to materials that are harmless and sustainable, like clay suspended in water or in transparent recyclable polymers or biopolymers. The latter are chemical compounds that are made up of chain-like molecules.
“The physical color mechanism isn’t yet used very widely by industry. The chemical mechanism dominates,” Fossum said. “The reason is that we haven’t yet found a way to fabricate structural colors that is fast, efficient and practical for industry.”
But that hurdle won’t last forever.
“Our findings open up a much more extensive use of nature’s way of making colors, instead of the toxic and unsustainable way we humans have been doing,” Fossum said.
That’s why this research is important, not only for producing paints or varnishes but also for use in cosmetics, food packaging and in several other areas.
The new method is now being patented in collaboration with NTNU Technology Transfer (TTO).
You can read more about this research and much more on the webpage for the Soft and Complex Matter Lab, where Fossum and Michels-Brito work.
Reference: Bright, non-iridescent structural coloration from clay mineral nanosheet suspensions, Science Advances (2022).DOI: 10.1126/sciadv.abl8147