It can pogo-stick along at 50-plus miles per hour, leaping 30-odd feet in a single bound. But that platinum-medal athleticism falls by the wayside at a sub-Saharan riverside, the source of life and death for the skittish impala stilling itself for a drink in 100-degree heat.
A Nile crocodile has silently baptized itself in that same muddy river for the past hour. When the unseen apex predator lashes from the water to seize the impala, its infamous teeth latch onto a hindquarter, jaws clenching with 5,000 pounds of force. Yet it’s the water itself that does the killing, with the deep-breathed reptile dragging its prey to the deep end to drown.
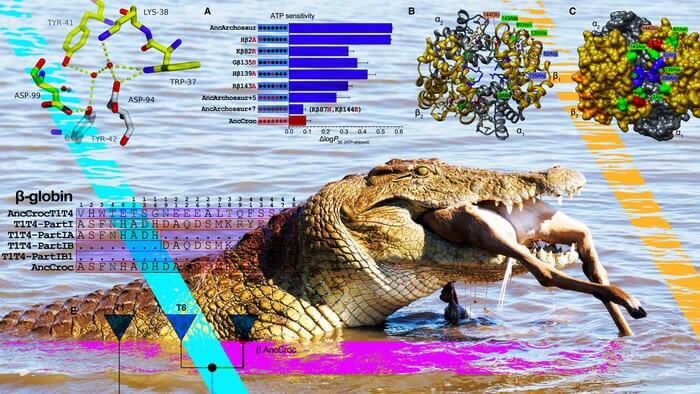
The success of the croc’s ambush lies in the nanoscopic scuba tanks — hemoglobins — that course through its bloodstream, unloading oxygen from lungs to tissues at a slow but steady clip that allows it to go hours without air. The hyper-efficiency of that specialized hemoglobin has led some biologists to wonder why, of all the jawed vertebrates in all the world, crocodilians were the lone group to hit on such an optimal solution to making the most of a breath.
By statistically reconstructing and experimentally resurrecting the hemoglobin of an archosaur, the 240-million-year-old ancestor of all crocodilians and birds, the University of Nebraska–Lincoln’s Jay Storz and colleagues have gleaned new insights into that why. Rather than requiring just a few key mutations, as earlier research suggested, the unique properties of crocodilian hemoglobin stemmed from 21 interconnected mutations that litter the intricate component of red blood cells.
That complexity, and the multiple knock-on effects that any one mutation can induce in hemoglobin, may have forged an evolutionary path so labyrinthine that nature failed to retrace it even over tens of millions of years, the researchers said.
“If it was such an easy trick — if it was that easy to do, just making a few changes — everyone would be doing it,” said Storz, a senior author of the study and Willa Cather Professor of biological sciences at Nebraska.
All hemoglobin binds with oxygen in the lungs before swimming the bloodstream and eventually releasing that oxygen to the tissues that depend on it. In most vertebrates, hemoglobin’s affinity for capturing and holding oxygen is dictated largely by molecules known as organic phosphates, which, by attaching themselves to the hemoglobin, can coax it into releasing its precious cargo.
But in crocodilians — crocodiles, alligators and their kin — the role of organic phosphates was supplanted by a molecule, bicarbonate, that is produced from the breakdown of carbon dioxide. Because hardworking tissues produce lots of carbon dioxide, they also indirectly generate lots of bicarbonate, which in turn encourages hemoglobin to dispense its oxygen to the tissues most in need of it.
“It’s a super-efficient system that provides a kind of slow-release mechanism that allows crocodilians to efficiently exploit their onboard oxygen stores,” Storz said. “It’s part of the reason they’re able to stay underwater for so long.”
As postdoctoral researchers in Storz’s lab, Chandrasekhar Natarajan, Tony Signore and Naim Bautista had already helped decipher the workings of the crocodilian hemoglobin. Alongside colleagues from Denmark, Canada, the United States and Japan, Storz’s team decided to embark on a multidisciplinary study of how the oxygen-ferrying marvel came to be.
Prior efforts to understand its evolution involved incorporating known mutations into human hemoglobin and looking for any functional changes, which were usually scant. Recent findings from his own lab had convinced Storz that the approach was flawed. There were plenty of differences, after all, between human hemoglobin and that of the ancient reptilian creatures from which modern-day crocodilians evolved.
“What’s important is to understand the effects of mutations on the genetic background in which they actually evolved, which means making vertical comparisons between ancestral and descendant proteins, rather than horizontal comparisons between proteins of contemporary species,” Storz said. “By using that approach, you can figure out what actually happened.”
So, with the help of biochemical principles and statistics, the team set out to reconstruct hemoglobin blueprints from three sources: the 240-million-year-old archosaur ancestor; the last common ancestor of all birds; and the 80-million-year-old shared ancestor of contemporary crocodilians. After putting all three of the resurrected hemoglobins through their paces in the lab, the team confirmed that only the hemoglobin of the direct crocodilian ancestor lacked phosphate binding and boasted bicarbonate sensitivity.
Comparing the hemoglobin blueprints of the archosaur and crocodilian ancestors also helped identify changes in amino acids — essentially the joints of the hemoglobin skeleton — that may have proved important. To test those mutations, Storz and his colleagues began introducing certain croc-specific mutations into the ancestral archosaur hemoglobin. By identifying the mutations that made archosaur hemoglobin behave more like that of a modern-day crocodilian, the team pieced together the changes responsible for those unique, croc-specific properties.
Counter to conventional wisdom, Storz and his colleagues discovered that evolved changes in hemoglobin’s responsiveness to bicarbonate and phosphates were driven by different sets of mutations, so that the gain of one mechanism was not dependent on the loss of the other. Their comparison also revealed that, though a few mutations were enough to subtract the phosphate-binding sites, multiple others were needed to eliminate phosphate sensitivity all together. In much the same way, two mutations seemed to directly drive the emergence of bicarbonate sensitivity — but only when combined with or preceded by other, easy-to-miss mutations in remote regions of the hemoglobin.
Storz said the findings speak to the fact that a combination of mutations might yield functional changes that transcend the sum of their individual effects. A mutation that produces no functional effect on its own might, in any number of ways, open a path to other mutations with clear, direct consequences. In the same vein, he said, those later mutations might influence little without the proper stage-setting predecessors already in place. And all of those factors can be supercharged or waylaid by the environment in which they unfold.
“When you have these complex interactions, it suggests that certain evolutionary solutions are only accessible from certain ancestral starting points,” Storz said. “With the ancestral archosaur hemoglobin, you have a genetic background that makes it possible to evolve the unique properties that we see in hemoglobins of modern-day crocodilians. By contrast, with the ancestor of mammals as a starting point, it may be that there’s some way that you could evolve the same property, but it would have to be through a completely different molecular mechanism, because you’re working within a completely different structural context.”
For better or worse, Storz said, the study also helps explain the difficulty of engineering a human hemoglobin that can mimic and approach the performance of the crocodilian.
“We can’t just say, ‘OK, it’s mainly due to these five mutations. If we take human hemoglobin and just introduce those mutations, voilà, we’ll have one with those same exact properties, and we’ll be able to stay underwater for two hours, too,’” Storz said. “It turns out that’s not the case.
“There are lots of can’t-get-there-from-here problems in the tree of life.”
Storz, Natarajan and Bautista conducted the study with Signore, now at the University of Manitoba; Aarhus University’s Angela Fago; Mississippi State University’s Federico Hoffmann; and Yokohama City University’s Jeremy Tame. The researchers detailed their findings in the journal Current Biology, receiving support from the National Science Foundation and National Institutes of Health.