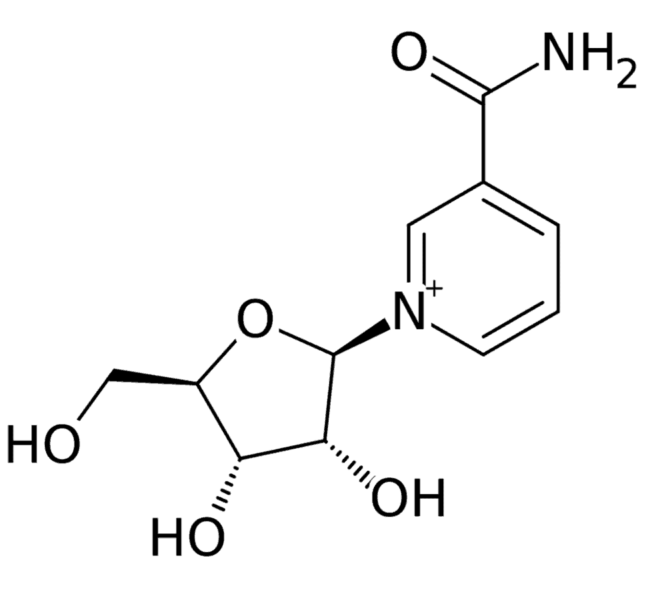
For the first time, a researcher at the University of Delaware College of Health Sciences in collaboration with a team at the National Institute on Aging, a division of the National Institutes of Health, has determined that the naturally occurring dietary supplement, nicotinamide riboside (NR), can enter the brain.
The discovery was made by Christopher Martens, assistant professor of kinesiology and applied physiology and director of the Delaware Center for Cognitive Aging Research, and Dr. Dimitrios Kapogiannis, a senior investigator at the National Institute on Aging. The finding is significant because it supports the idea that NR, upon reaching the brain, can alter the metabolism of relevant biological pathways involved in neurodegenerative diseases like Alzheimer’s. Their work, supported by an NIH grant, and in part by the Intramural Research Program of the NIH National Institute on Aging, was recently published in the journal Aging Cell.
Upon consumption, NR is readily converted into nicotinamide adenine dinucleotide (NAD+), which is critical to cellular repair and the repair of damaged DNA.
“NAD+ is gradually lost as we get older or develop chronic diseases. Loss of NAD+ is linked to obesity and other negative lifestyle habits like smoking,” Martens said. “Because more NAD+ is needed to counteract those negative consequences, it’s more likely to be depleted in the face of negative lifestyle habits.”
Martens has been studying the compound since he was a postdoctoral fellow at the University of Colorado Boulder. In an initial study, he found that levels of NAD+ could be boosted in the blood if people ingested NR, but it was not clear if it could reach other tissues in the body.
“We had some preliminary signs of efficacy, including lower blood pressure in people who had high blood pressure to begin with,” he said. “But until now, it was unknown whether NR reached targeted organs like the brain to have a real therapeutic effect.”
Measuring the level of NAD+ in the brain in humans is challenging. There are emerging techniques involving MRI, but these only provide an indirect measure and are costly and difficult to perform. Instead, Martens and colleagues measured NAD+ directly in tiny particles called extracellular vesicles that originated from neurons and ended up in the blood. These extracellular vesicles can provide cutting-edge blood-based biomarkers for brain disorders and serve as a “liquid biopsy” of neurons, giving researchers a rare look at what’s inside them.
“Each vesicle has a unique molecular signature on its surface, including proteins that give you clues about its origin,” Martens said. “In our case, we selected vesicles that carry markers that are characteristic of neurons, and so we have confidence that the NAD+ we measured in them reflects what happens in the neurons, and by extension the brain.”
Using samples from their first initial clinical trial, the researchers determined, first, that NAD+ levels went up in these vesicles after six weeks.
“When NAD+ goes up in these vesicles, we see an association with some of the biomarkers of neurodegenerative disease,” Martens said. “Particularly, in people where we saw an increase in NAD+, we also saw changes in biomarkers like amyloid beta and tau, which are both related to Alzheimer’s disease,” Martens said.
Martens and Kapogiannis also found a correlation between these neurodegenerative biomarkers and change in NAD+.
“If NAD+ went up a lot, there was typically a larger change in some of the disease biomarkers,” Martens said. “That tells us the NAD+ is not only getting into the brain but it’s likely also having some effect on its metabolism and multiple interrelated pathways.”
Some of these blood-based biomarkers could be used down the road to determine if NAD+ depletion is a cause of Alzheimer’s and other neurodegenerative diseases. It is even possible that these types of tests could become more accessible to the population for more routine testing.
Martens is leading a 12-week study involving NR in older adults with mild cognitive impairment. The study is supported by the Delaware Center for Cognitive Aging Research and the National Institute on Aging and is actively seeking more participants.
Through the study, Martens seeks to determine whether increased consumption of NR has an even larger effect in people with cognitive impairment.
“They’re coming in with cognitive deficits, and as a result, are more likely to have an accumulation of some of these biomarkers in their brain, so there’s a chance we’ll see bigger reductions in these biomarkers because they have more of them in their cells,” Martens said.
Nearly all drugs on the market for patients with Alzheimer’s have only a modest effect on the symptoms but do not significantly stop the underlying progression of the disease.
“In our ongoing trial, we’re measuring markers of cognitive function and other things related to functional independence and quality of life, but we’re also hoping to gain some insight on the underlying disease process,” Martens said. “We’re hoping that the people who take the NR might have preserved function.”
After proving its efficacy, Martens and Kapogiannis will test whether increased use of NR improves cognition, and ultimately, whether it can be used to slow neurodegenerative disease progression.
“We were among the first to do a chronic dosing study in humans, and as a result, we’ve been at the forefront of this field for a few years,” Martens said. “Now, we’re at a turning point, where we can start to determine whether NAD+ increases in other tissues as well, and that’s likely where the more important signal will be in terms of resolving disease.”
The Delaware Center for Cognitive Aging Research relies on the support of individuals and organizations to accelerate interdisciplinary research, education and clinical training in Alzheimer’s disease and related disorders. If you are interested in learning more about the Center or how you can support this important research, please contact Doug Kleintop at 302-831-0612 or [email protected].