Scientists have long speculated about the physical changes that occur in the brain when a new memory is formed. Now, research from the National Institute for Physiological Sciences (NIPS) has shed light on this intriguing neurological mystery.
In a study recently published in Nature Communications, The research team has succeeded in detecting the brain neuronal networks involved in trauma memory by using a novel method that combines optical and machine-learning-based approaches, capturing the complex changes that occur during memory formation and uncovering the mechanisms by which trauma memories are created.
Animals learn to adapt to changing environments for survival. Associative learning, which includes classical conditioning, is one of the simplest types of learning and has been studied intensively over the past century. During the last two decades, technical developments in molecular, genetic, and optogenetic methods have made it possible to identify brain regions and specific populations of neurons that control the formation and retrieval of new associative memories. For instance, the dorsal part of the medial prefrontal cortex (dmPFC) is critical for the retrieval of associative fear memory in rodents. However, the way in which the neurons in this region encode and retrieve associative memory is not well understood, which the research team aimed to address.
“The dmPFC shows specific neural activation and synchrony during fear-memory retrieval and evoked fear responses, such as freezing and heart rate deceleration,” explains lead author Masakazu Agetsuma. “Artificial silencing of the dmPFC in mice suppressed fear responses, indicating that this region is required to recall associative fear-memory. Because it is connected with brain systems implicated in learning and associated psychiatric diseases, we wanted to explore how changes in the dmPFC specifically regulate new associative memory information.”
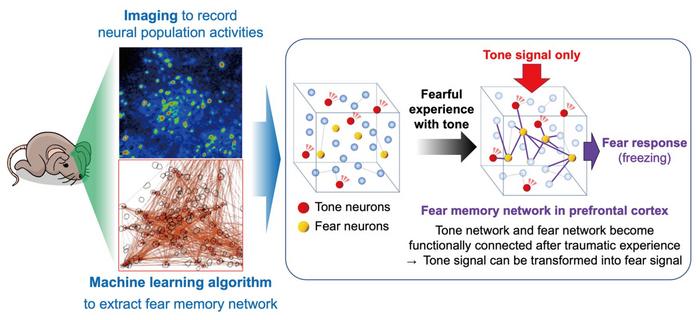
The research team used longitudinal two-photon imaging and various computational neuroscience techniques to determine how neural activity changes in the mouse prefrontal cortex after learning in a fear-conditioning paradigm. Prefrontal neurons behave in a highly complex manner, and each neuron responds to various sensory and motor events. To address this complexity, the research team developed a new analytical method based on the ‘elastic net,’ a machine-learning algorithm, to identify which specific neurons encode fear memory. They further analyzed the spatial arrangement and functional connectivity of the neurons using graphical modeling.
“We successfully detected a neural population that encodes fear memory,” says Agetsuma. “Our analyses showed us that fear conditioning induced the formation of a fear-memory neural network with ‘hub’ neurons that functionally connected the memory neurons.”
Importantly, the researchers uncovered direct evidence that associative memory formation was accompanied by a novel associative connection between originally distinct networks, i.e., the conditioned stimulus (CS, e.g., tone) network and the unconditioned stimulus (US, e.g., fearful experience) network. “We propose that this newly discovered connection might facilitate information processing by triggering a fear response (CR) to a CS (i.e., a neural network for CS-to-CR transformation).”
Memories have long been thought to be formed by the enhancement of neural connections, which are strengthened by the repeated activation of groups of neurons. The findings of the present study, which were based on both real-life observations and model-based analysis, support this. Furthermore, the study demonstrates how combined methods (optics and machine learning) can be used to visualize the dynamics of neural networks in great detail. These techniques could be used to uncover additional information about the neurological changes associated with learning and memory.
The article, “Activity-dependent organization of prefrontal hub-networks for associative learning and signal transformation” was published in Nature Communications at DOI:10.1038/s41467-023-41547-5