In new research physicists have wielded the power of chess to design a group of intricate mazes, which could ultimately be used to tackle some of the world’s most pressing challenges.
Their unique labyrinthine creations, inspired by the Knight’s movements on a chessboard, might help unravel other notoriously difficult problems including simplifying industrial processes from carbon capture to fertiliser production.
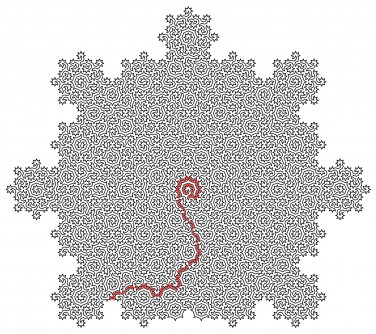
Lead author Dr Felix Flicker, Senior Lecturer in Physics at the University of Bristol, said: “When we looked at the shapes of the lines we constructed, we noticed they formed incredibly intricate mazes. The sizes of subsequent mazes grow exponentially – and there are an infinite number of them.”
In a Knight’s tour, the chess piece (which jumps two squares forwards and one to the right) visits every square of the chessboard just once before returning to its starting square. This is an example of a ‘Hamiltonian cycle’ – a loop through a map visiting all stopping points only once.
The theoretical physicists, led by the University of Bristol, constructed an infinity of ever-larger Hamiltonian cycles in irregular structures which describe exotic matter known as quasicrystals.
The atoms in quasicrystals are arranged differently to those in crystals such as salt or quartz. Whereas the atoms in crystals repeat at regular intervals, like the squares of a chessboard, quasicrystal atoms do not. Instead, they do something rather more mysterious: quasicrystals can be described mathematically as slices through crystals that live in six dimensions, as opposed to the three of our familiar universe.
Only three natural quasicrystals have ever been found, all in the same Siberian meteorite. The first artificial quasicrystal was created accidentally in the 1945 Trinity Test, the atomic bomb explosion dramatised in the film Oppenheimer.
The group’s Hamiltonian cycles visit every atom on the surface of certain quasicrystals precisely once. The resulting paths form uniquely complex mazes, described by mathematical objects called ‘fractals’.
These paths have the special property that an atomically sharp pencil could draw straight lines connecting all neighbouring atoms, without the pencil lifting or the line crossing itself. This has applications in a process known as ‘scanning tunneling microscopy’, where the pencil is an atomically sharp microscope tip capable of imaging individual atoms. The Hamiltonian cycles form the fastest possible routes for the microscope to follow. This is helpful, as a state-of-the-art scanning tunneling microscopy image can take a month to produce.
The problem of finding Hamiltonian cycles in general settings is so hard that its solution would automatically solve many important problems yet to be overcome in the mathematical sciences.
Dr Flicker added: “We show that certain quasicrystals provide a special case in which the problem is unexpectedly simple. In this setting, we therefore render some seemingly-impossible problems tractable. This could include practical purposes spanning different realms of science.”
For example, ‘adsorption’ is a key industrial process in which molecules stick to the surfaces of crystals. So far, only crystals are used for adsorption industrially. If the atoms of a surface admit a Hamiltonian cycle, flexible molecules of the right size can pack with perfect efficiency by lying along these atomic mazes. The research results demonstrate that quasicrystals can be highly efficient adsorbers. One use of adsorption is carbon capture and storage, in which CO2 molecules are stopped from entering the atmosphere.
Co-author Shobhna Singh, a PhD researcher in Physics at Cardiff University, said:“Our work also shows quasicrystals may be better than crystals for some adsorption applications. For example, bendy molecules will find more ways to land on the irregularly arranged atoms of quasicrystals. Quasicrystals are also brittle, meaning they readily break into tiny grains. This maximises their surface area for adsorption.”
Efficient adsorption could also make quasicrystals surprising candidates for catalysts, which increase industrial efficiency by lowering the energy of chemical reactions. For example, adsorption is a key step in the Haber catalysis process, used to produce ammonia fertiliser for farming.