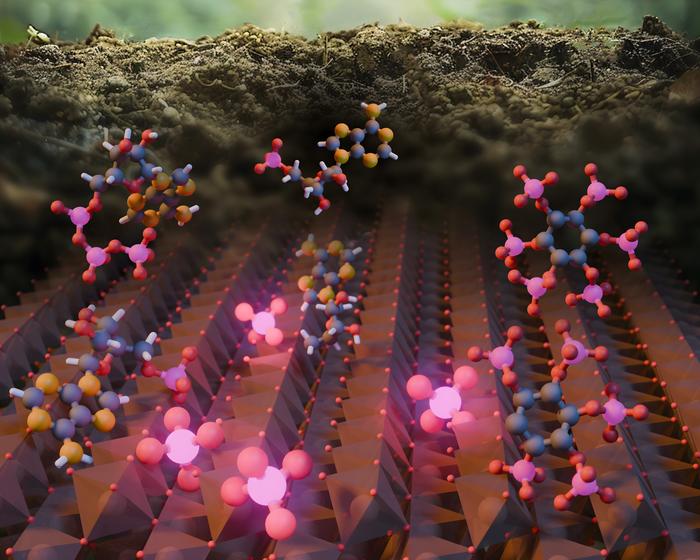
A team of researchers led by Northwestern University has uncovered a new pathway in Earth’s phosphorus cycle, revealing that iron oxide minerals play a crucial role in transforming organic phosphorus into its inorganic form. This discovery, published in Nature Communications, challenges long-held assumptions about how this essential nutrient is recycled in nature.
The Hidden Role of Iron Oxide in Phosphorus Recycling
Phosphorus is a vital nutrient for plant growth and a key component of fertilizers. Until now, scientists believed that only enzymes from plants and microbes could break down organic phosphorus into the inorganic form that plants can use. However, this new study shows that iron oxide, a common mineral in soils and sediments, can perform this transformation just as effectively.
Ludmilla Aristilde, associate professor of environmental engineering at Northwestern’s McCormick School of Engineering, who led the research, explains: “Currently, the main source of phosphorus for fertilizers is from mining. It’s a finite resource that we eventually will run out of. According to some estimates, we might run out in as soon as 50 years or in a couple hundred years. We are looking into ways to leverage nature-based solutions for phosphorus recycling because we cannot have food security without it.”
The team’s laboratory experiments revealed that iron oxide minerals can cleave phosphorus from nucleotides – structural components of DNA and RNA – in decaying organic matter. Surprisingly, they found that this mineral-driven process occurs at rates comparable to those reported for soil enzymes.
Unraveling the Mystery of Missing Phosphorus
The researchers used a specialized X-ray technique at the Stanford Synchrotron Radiation Lightsource to solve a puzzling aspect of their findings. While they detected transformation products in the solution, a significant portion of the inorganic phosphorus was missing.
“Lo and behold, we found that the phosphorus was clinging to the surface of the iron oxide,” Aristilde said. “Basically, the minerals can recycle phosphorus from DNA and RNA molecules. But not all organic phosphorus is released in the solution because it is stuck to the surface.”
This discovery explains why previous studies may have underestimated the role of minerals in phosphorus cycling. It also highlights the complexity of nutrient dynamics in soil systems.
Why it matters: This research has significant implications for our understanding of global nutrient cycles and could lead to more sustainable agricultural practices. By revealing a new natural mechanism for phosphorus recycling, it opens up possibilities for developing nature-based solutions to address the looming phosphorus shortage.
The study also raises intriguing questions about the potential for past or present life on other planets. Aristilde notes, “Mars is red because it’s full of iron oxides. If there is inorganic phosphorus found to be trapped in them, it is reasonable to ask: ‘Could this phosphorus have an organic origin from life?'”
As we face the challenges of feeding a growing global population and the eventual depletion of phosphorus reserves, this research provides valuable insights that could inform future strategies for sustainable nutrient management. It underscores the importance of considering both biological and geological processes in our understanding of Earth’s complex nutrient cycles.
Looking ahead, this discovery opens up new avenues for research into sustainable agriculture and environmental management. Future studies may explore how to harness this mineral-driven process to enhance phosphorus recycling in agricultural soils or develop new technologies for phosphorus recovery from waste streams.
As our understanding of Earth’s phosphorus cycle continues to evolve, it’s clear that the interplay between biology and geology is more intricate than previously thought. This research not only expands our knowledge of fundamental Earth processes but also provides a foundation for developing innovative solutions to pressing environmental challenges.