Summary: Researchers have discovered how a bacterial parasite thrives inside the nuclei of deep-sea mussels, using host-acquired genes to prevent cell death and sustain its massive reproduction.
Estimated reading time: 7 minutes
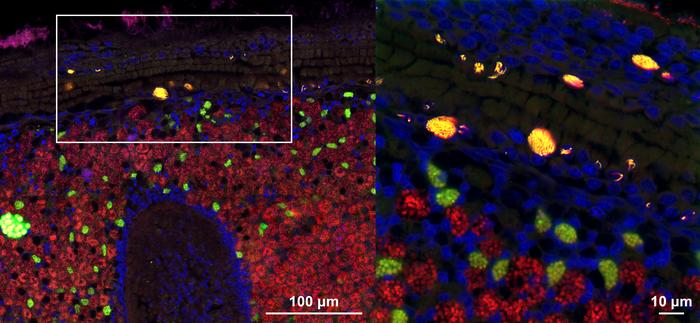
A team of scientists from the Max Planck Institute for Marine Microbiology has uncovered the remarkable strategies employed by a bacterial parasite that inhabits the cell nuclei of deep-sea mussels. This groundbreaking study, published in Nature Microbiology, provides the first in-depth analysis of an intranuclear parasite in animals, revealing surprising tactics for survival and reproduction.
The parasite, named Candidatus Endonucleobacter, infects mussels found near hydrothermal vents and cold seeps in the deep ocean. What makes this bacterium extraordinary is its ability to enter the nucleus – the command center of the cell – and replicate to staggering numbers without immediately killing its host.
Why it matters: This research not only expands our understanding of host-microbe interactions but also has potential implications for fields ranging from evolutionary biology to cancer research. The unusual survival mechanisms employed by Ca. Endonucleobacter could provide new insights into how pathogens evade host immune responses and how cells regulate their life-and-death processes.
A Parasite’s Balancing Act
The researchers faced a puzzling question: How does Ca. Endonucleobacter multiply so extensively inside a cell’s nucleus without triggering immediate cell death? Dr. Niko Leisch, co-senior author of the study, explains the challenge:
“We wanted to understand how the bacterium infects and reproduces inside nuclei, and in particular how these bacteria acquire the nutrients they need for their massive replication, yet keep their host cells from dying.”
Using a combination of advanced molecular and imaging techniques, the team made several key discoveries:
- Nutrient acquisition: Unlike many intranuclear bacteria, Ca. Endonucleobacter doesn’t digest its host’s DNA or RNA. Instead, it feeds on sugars, lipids, and other cellular components. This strategy allows the host cell to function long enough for the parasite to complete its extensive reproduction cycle.
- Apoptosis inhibition: The bacteria produce proteins called inhibitors of apoptosis (IAPs) to prevent the host cell from initiating its self-destruct sequence. This is a common defense mechanism that cells use when infected or damaged.
- Gene transfer from host to parasite: Surprisingly, the genes for these IAPs appear to have been acquired from the mussel host through horizontal gene transfer – a rare occurrence of genetic material moving from a complex organism to a bacterium.
An Arms Race Within the Cell
The relationship between Ca. Endonucleobacter and its host cell can be described as a molecular arms race. As Miguel Ángel González Porras, the study’s first author, explains:
“Interestingly, these bacteria have come up with a sophisticated strategy to keep their host cells from killing themselves. They produce proteins that suppress apoptosis called inhibitors of apoptosis (IAPs).”
As the infection progresses, both the parasite and the host cell ramp up their efforts. The bacteria produce more IAPs, while the host cell increases its production of proteins that promote apoptosis. Eventually, after the parasite has multiplied sufficiently, the host cell ruptures, releasing the bacteria to infect new cells.
Evolutionary Implications and Future Research
The discovery of IAPs in Ca. Endonucleobacter is particularly significant because these proteins were previously known only in animals and some viruses. Dr. Nicole Dubilier, co-senior author, highlights the evolutionary importance of this finding:
“The discovery of IAPs in Ca. Endonucleobacter was one of the most surprising results of our study, because these proteins are only known from animals and a few viruses, but have never been found in bacteria.”
This unexpected gene transfer from a complex organism to a bacterium challenges our understanding of how microbes evolve and acquire new traits. It suggests that horizontal gene transfer from eukaryotes (complex cells) to prokaryotes (simple cells) may be more common than previously thought.
Broader Implications for Science and Medicine
The insights gained from this study extend beyond the realm of marine biology. Dr. Leisch outlines some potential applications:
“Our research sheds light on an overlooked mechanism of genetic exchange — HGT from eukaryotes to bacteria — potentially influencing how we understand microbial evolution and pathogenesis. Moreover, our study offers insights into apoptosis regulation, which is relevant to cancer research and cell biology.”
Understanding how Ca. Endonucleobacter manipulates its host’s cellular machinery could provide new perspectives on:
- Parasitic infections and immune evasion strategies in other organisms
- The regulation of apoptosis, which is crucial in cancer research
- The evolution of symbiotic relationships between bacteria and complex organisms
As researchers continue to explore the intricate world of host-microbe interactions, the story of Ca. Endonucleobacter serves as a reminder of nature’s complexity and the potential for discovery in even the most extreme environments.
Quiz:
- What is the primary food source for Candidatus Endonucleobacter inside the host cell? a) Host cell DNA b) Host cell RNA c) Sugars and lipids from the host cell d) Other bacteria in the cell
- How does Ca. Endonucleobacter prevent the host cell from undergoing apoptosis? a) By producing inhibitors of apoptosis (IAPs) b) By hiding from the host cell’s immune system c) By quickly killing the host cell d) By repairing damaged host DNA
- What surprising discovery did the researchers make about the origin of the IAP genes in Ca. Endonucleobacter? a) They evolved independently in the bacteria b) They were acquired from viruses c) They were acquired from the mussel host through horizontal gene transfer d) They are common in all bacteria
Answer Key:
- c) Sugars and lipids from the host cell
- a) By producing inhibitors of apoptosis (IAPs)
- c) They were acquired from the mussel host through horizontal gene transfer