Any medical device implanted in the body attracts bacteria, proteins, and other microbes to its surface, causing infections and thrombosis (blood clotting) that lead to hundreds of thousands of deaths annually. Devices can be coated with antibiotics, blood thinners, and other agents — but these eventually dissolve, limiting their longevity and effectiveness.
Now, Semprus BioSciences, a startup co-founded by two MIT alumni — Christopher Loose PhD ’07 and CEO David Lucchino MBA ’06 — is developing a novel biomaterial for implanted medical devices that permanently barricades these troublesome microbes from the device’s surface.
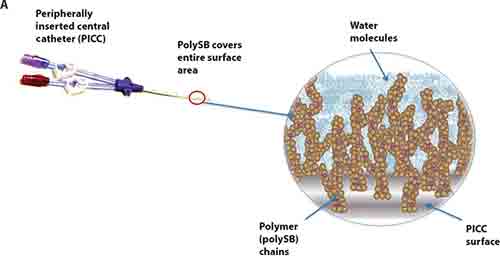
The biomaterial is a nonleaching polymeric sulfobetaine (polySB) that, when applied to a medical device, sprouts a thicket of polymers that attract water, creating an impenetrable barrier for microbes. Its chemical makeup also mimics that of cells important to homeostasis, potentially reducing the body’s natural rejection of implanted devices.
“Basically, we’ve developed a long-lasting solution that blocks negative consequences in the body by making devices look more like the human body,” Loose says.
The application of polySB to catheters yields a significant reduction in the buildup of protein, mammalian cells, and microbes on a device’s surface, compared with unmodified catheters. This has potential to reduce blood clots and infection, and improve overall patient health, the co-founders say.
The technology was described in a paper published last year in the journal Science Translation Medicine, co-authored by Loose, Lucchino, MIT Institute Professor Robert Langer, and other researchers.
Based on Loose’s work at MIT, the biomaterial has positioned Semprus as a fast-growing biotech firm in Kendall Square. In its six years, the startup — seed-funded, in part, by the MIT $100K Entrepreneurship Competition — has earned millions of dollars in private and federal funding. In 2012, Semprus sold to a medical device-manufacturing giant for an amount that could reach $80 million. As a wholly owned subsidiary, the Semprus team continues developing the technology.
Semprus’ first commercial product based on the biomaterial, Semprus Sustain technology, is designed specifically for venous catheters and recently earned clearance from the Food and Drug Administration as a medical device deemed safe and effective for commercial distribution in the United States. It also recently received designation as a product meeting European Union standards of health, safety, and environmental protection.
Addressing an ‘unmet need’
The Semprus story began in Langer’s lab, where Loose, a chemical engineering PhD student, was charged with developing medical devices that could permanently be inserted in the body without triggering an immune response — in other words, creating medical devices that “looked more human,” Loose says.
Loose developed a means of applying naturally occurring antibiotics, called antimicrobial peptides — found in bacteria and human sweat — on medical devices. These peptides would puncture bacteria that came near, and microbes would have trouble developing resistance to them. In 2007, Loose was named one of “35 innovators under 35” by MIT Technology Review for this innovation.
Seeing commercial potential, Langer — a chemical engineer, bioengineer, and famed MIT entrepreneur — “played matchmaker” between Loose and Lucchino, who had previously worked for Polaris Ventures Partners and was, at the time, an Alfred P. Sloan Fellow at the MIT Sloan School of Management.
The two had an instant rapport, Lucchino says — and an ambition to commercialize Loose’s innovation. So they “went to school” on the medical-device market, canvassing hospitals to meet patients and to talk with nurses and doctors about unmet clinical needs.
“We learned quickly that the most successful entrepreneurs are good listeners,” Lucchino says. “We conducted our own ‘listening tour’ to understand the problem, so we could develop the most strategic business and technology solution.”
They found many patients suffering from chronic diseases — such as diabetes, cancer, and heart disease — and a lack of permanent “coatings” for medical implants that might help these patients. Thus, Loose says, they were ahead of the curve in addressing the “unmet need” of the medical devices market.
“We realized an unmet need that was going to grow over the next few years and we were one of the first to have a solution to it,” he says. “Everything starts with the unmet need.”
Today, the Semprus technology has proven its effectiveness. In the Science Translation Medicine paper, the co-founders exposed polySB-modified catheters to blood for 60 days. In vitro, the modified catheters — on both their external and internal surfaces — saw a 98 percent reduction in the accumulation of platelets and three types of white blood cells. Additionally, thrombotic material on the device was reduced by 99 percent. In vivo, the modified catheters showed a 99 percent reduction in thrombus accumulation, 50 percent less inflammation, and fewer bacteria.
‘A pathway’ for a startup
A catalyst for starting Semprus was winning MIT’s $100K (as SteriCoat) in 2006, and going on to win similar business-plan competitions at Harvard and Oxford universities.
“Once we had clear confirmation that there was an enormous unmet need, in terms of cost and patient impact, we had a clear business plan refined through the competitions, and even more so thereafter,” Loose says. “It gave us a pathway to say, ‘This is how we can solve a big problem and here’s the pathway to do it.’”
The two were also “very diligent in going to any networking and entrepreneur event at MIT. There is a tremendous [number] of MIT alumni, very open with their time, who provided critical support and advice,” Loose says.
Through MIT’s network of entrepreneurs, investors, and lawyers, “we were able to assemble a great team of advisers to refine our plans and give us the momentum to go out and do financing,” Lucchino says.
Under Lucchino’s stewardship, Semprus secured $28.5 million in venture capital financing and $2.4 million in federal funding, primarily from the National Science Foundation, and grew from two to 40 employees.
Lucchino says he owes some of his business acumen to his education at MIT Sloan, which taught him a broad set of entrepreneurial skills in finance, business, and operations strategies. “It was continuing to fertilize my entrepreneurial soil to get me in the best position to succeed,” Lucchino says.
Today, the two entrepreneurs continue to mentor students and give talks at MIT and Harvard Business School, sharing startup advice, or their “rules of the road” — such as knowing your technological and personal limitations, working with limited resources, being flexible to economic and other changes, and, most importantly, teamwork.
“No one person builds a company alone,” says Lucchino, who has served as guest lecturer at MIT Sloan. “As your company grows and there’s real value attached to what you’re doing, you need to be able to trust the people you’re working with. Chris and I, as a team, made Semprus a success. The most important skill and functionality we have is trust.”


Patent translation is a very complicated process.Translation services usa besides mastering the source and target languages, the translator must be highly knowledgeable in that technical field which is closely related to the patent subject. A high accuracy is required during the translation of the patents. It would be great if you hired for your translation project a current or retired patent agent, because they know exactly how the language shall be structured. Another important factor in the patent translation is to hire a translator who is certified and native speaker of the target language. The translation itself shall be certified if it is meant to be used for official purposes.