A new drug target to fight Alzheimer’s disease has been discovered by a research team led by Gong Chen, a Professor of Biology and the Verne M. Willaman Chair in Life Sciences at Penn State University. The discovery also has potential for development as a novel diagnostic tool for Alzheimer’s disease, which is the most common form of dementia and one for which no cure has yet been found. A scientific paper describing the discovery will be published in Nature Communications on 13 June 2014.
Chen’s research was motivated by the recent failure in clinical trials of once-promising Alzheimer’s drugs being developed by large pharmaceutical companies. “Billions of dollars were invested in years of research leading up to the clinical trials of those Alzheimer’s drugs, but they failed the test after they unexpectedly worsened the patients’ symptoms,” Chen said. The research behind those drugs had targeted the long-recognized feature of Alzheimer’s brains: the sticky buildup of the amyloid protein known as plaques, which can cause neurons in the brain to die. “The research of our lab and others now has focused on finding new drug targets and on developing new approaches for diagnosing and treating Alzheimer’s disease,” Chen explained.
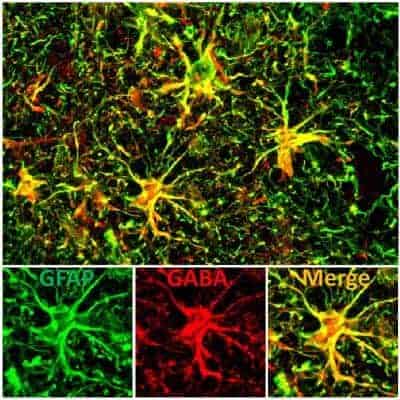
“We recently discovered an abnormally high concentration of one inhibitory neurotransmitter in the brains of deceased Alzheimer’s patients,” Chen said. He and his research team found the neurotransmitter, called GABA (gamma-aminobutyric acid), in deformed cells called “reactive astrocytes” in a structure in the core of the brain called the dentate gyrus. This structure is the gateway to hippocampus, an area of the brain that is critical for learning and memory.
Chen’s team found that the GABA neurotransmitter was drastically increased in the deformed versions of the normally large, star-shaped “astrocyte” cells which, in a healthy individual, surround and support individual neurons in the brain. “Our research shows that the excessively high concentration of the GABA neurotransmitter in these reactive astrocytes is a novel biomarker that we hope can be targeted in further research as a tool for the diagnosis and treatment of Alzheimer’s disease,” Chen said.
Chen’s team developed new analysis methods to evaluate neurotransmitter concentrations in the brains of normal and genetically modified mouse models for Alzheimer’s disease (AD mice). “Our studies of AD mice showed that the high concentration of the GABA neurotransmitter in the reactive astrocytes of the dentate gyrus correlates with the animals’ poor performance on tests of learning and memory,” Chen said. His lab also found that the high concentration of the GABA neurotransmitter in the reactive astrocytes is released through an astrocyte-specific GABA transporter, a novel drug target found in this study, to enhance GABA inhibition in the dentate gyrus. With too much inhibitory GABA neurotransmitter, the neurons in the dentate gyrus are not fired up like they normally would be when a healthy person is learning something new or remembering something already learned.
Importantly, Chen said, “After we inhibited the astrocytic GABA transporter to reduce GABA inhibition in the brains of the AD mice, we found that they showed better memory capability than the control AD mice. We are very excited and encouraged by this result, because it might explain why previous clinical trials failed by targeting amyloid plaques alone. One possible explanation is that while amyloid plaques may be reduced by targeting amyloid proteins, the other downstream alterations triggered by amyloid deposits, such as the excessive GABA inhibition discovered in our study, cannot be corrected by targeting amyloid proteins alone. Our studies suggest that reducing the excessive GABA inhibition to the neurons in the brain’s dentate gyrus may lead to a novel therapy for Alzheimer’s disease. An ultimate successful therapy may be a cocktail of compounds acting on several drug targets simultaneously.”