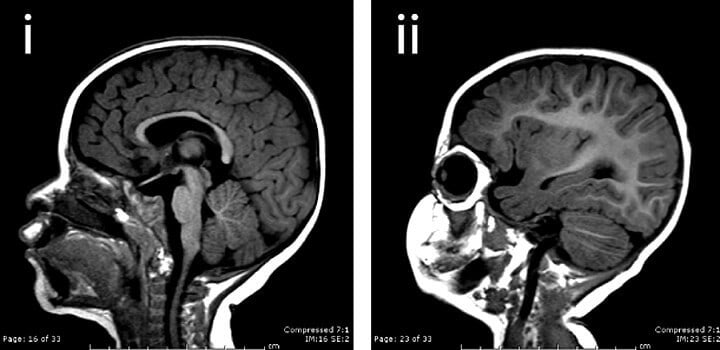
A new Yale-led study of children with neurodevelopmental abnormalities of the brain identifies a “cutting” enzyme crucial to the shaping and division of brain cells as well as the replenishment of neural stem cells.
The study, appearing online Dec. 17 in the journal Neuron, helps explain the molecular basis of complex brain abnormalities, including small brain size (microcephaly) observed in children who were suffering from a wide variety of clinical problems, ranging from severe cognitive deficits to autism spectrum disorders.
The Yale team was led by Ketu Mishra-Gorur and Ahmet Caglayan in the lab of Murat Gunel, the Nixdorff-German Professor and chair in the Department of Neurosurgery, and professor of genetics and neurobiology at Yale School of Medicine. The team sequenced all protein-coding genes (exome) of children with inherited cerebral cortex malformations at the Yale Center for Mendelian Genetics. In collaboration with Joseph Gleeson’s group at University of California-San Diego, they identified five harmful mutations in the KATNB1 gene, which encodes for a portion of the Katanin protein. Katanin, named after the Japanese sword, or katana, is an enzyme that severs microtubules that support the structure of cells and are crucial to their division.
The Gunel group showed that in diverse organisms ranging from zebrafish to fruit flies, silencing KATNB1 resulted in dramatically reduced brain size. The mutations severely impact asymmetric, rather than symmetric, cell division. Asymmetric cell division replenishes the neural stem cell population by producing both a “progenitor” and a “daughter” cell in each division. This allows for an exponential increase in cell numbers during early brain development, which is lost in brains with mutant KATNB1.
“It was generally understood that asymmetric cell division plays a vital role in cerebral cortical development,” Gunel said. “The discovery of KATNB1 mutations now reveals a beautiful example of the fundamental importance of this mechanism and how the loss of asymmetric cell division results in a small brain size.”
In an accompanying paper in Neuron, a team lead by Christopher Walsh of Harvard University also independently found that the lack of the same gene causes severe brain deformities in humans, mice, and zebrafish.
The work was funded by the Yale Program on Neurogenetics, the National Institutes of Health, Howard Hughes Medical Institute, and the Gregory M. Kiez and Mehmet Kutman Foundation.