Memory loss in older adults often raises the scary specter of Alzheimer’s disease — even if the dragging memory is just routine aging. And if Alzheimer’s is diagnosed, patients have no idea what to expect, and when.
But this big gap in clinical practice surrounding dementia may soon be closed. A research team at Weill Cornell Medical College and the University of California, San Francisco has developed a mathematical model that determines where in the brain Alzheimer’s has spread, predicts where it will appear next and how fast the brain’s atrophy patterns will change — giving patients a roadmap of their future.
It can also tell when mild cognitive impairment (MCI) — often believed to be a precursor to Alzheimer’s — is just simple memory loss, without further progressing to the Alzheimer phase.
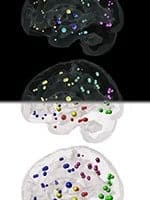
The model, published Jan. 15 in the journal Cell Reports, is based on the new understanding that the two toxic proteins that are the hallmarks of Alzheimer’s — tau and amyloid beta — spread in a neuron-to-neuron, prion-like fashion from the hippocampus, where the disease develops, to networks of neurons throughout the brain in a very predictable way.
“Neurologists today cannot tell a person with any certainty what a person with MCI or Alzheimer’s is going to experience in the future,” said lead author Dr. Ashish Raj, an associate professor of computer science in radiology at Weill Cornell. “This is a very debilitating problem if you are the person who is undergoing early signs of dementia and you want to know what will happen to you and when.
“With our model, we know exactly where Alzheimer’s will go, how fast it will go and which parts of the brain it will affect,” added Dr. Raj, who is also an associate professor of neuroscience at the Feil Family Brain and Mind Research Institute at Weill Cornell. “Because of this, we might be able to say with some confidence the time frame in which a person will convert to dementia or whether they will convert to dementia at all. This is very unique — and long needed.”
The team studied the brains of 418 living participants with diagnoses of Alzheimer’s or MCI, using a MRI brain atrophy scan and PET glucose metabolism scan, a test that looks at activity in the brain. Together, the scans provide a spatial map of brain atrophy in individual patients.
Dr. Raj expects that roadmaps of many neurodegenerative diseases, such as Parkinson’s, will soon be possible. He says the maps will be based on the new understanding that all types of dementia is caused by toxic proteins that spread from one neuron to the next one, in distinct patterns.
“We have long known that neurodegeneration is linked to one group of toxic proteins or another, but the discovery that these proteins spread, in a prion-like pattern, is relatively new,” Dr. Raj said. “That tells us that the trajectory of the neurodegenerative disease is purely a deterministic process — one that we can now use to inform our clinicians and patients.”