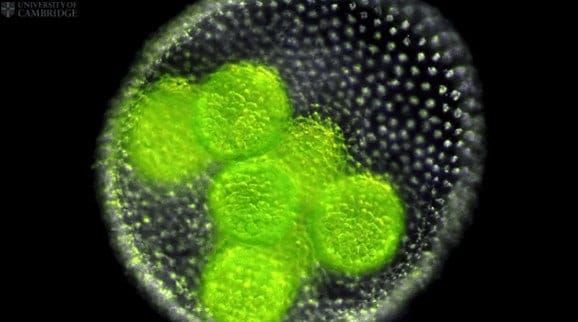
Researchers from the University of Cambridge have captured the first three-dimensional images of a live embryo turning itself inside out. The images, of embryos of a green alga called Volvox, make an ideal test case to understand how a remarkably similar process works in early animal development.
Using fluorescence microscopy to observe the Volvox embryos, the researchers were able to test a mathematical model of morphogenesis – the origin and development of an organism’s structure and form – and understand how the shape of cells drives the process of inversion, when the embryo turns itself from a sphere to a mushroom shape and back again. Their findings are published today (27 April) in the journal Physical Review Letters.
The processes observed in the Volvox embryo are similar to the process of gastrulation in animal embryos – which biologist Lewis Wolpert called “the most important event in your life.” During gastrulation, the embryo folds inwards into a cup-like shape, forming the primary germ layers which give rise to all the organs in the body. Volvox embryos undergo a similar process, but with an additional twist: the embryos literally turn themselves right-side out during the process.
Gastrulation in animals results from a complex interplay of cell shape changes, cell division and migration, making it difficult to develop a quantitative understanding of the process. However, Volvox embryos complete their shape change only by changing cell shapes and the location of the connections between cells, and this simplicity makes them an ideal model for understanding cell sheet folding.
In Volvox embryos, the process of inversion begins when the embryos start to fold inward, or invaginate, around their middle, forming two hemispheres. Next, one hemisphere moves inside the other, an opening at the top widens, and the outer hemisphere glides over the inner hemisphere, until the embryo regains its spherical shape. This remarkable process takes place over approximately one hour.
Previous work by biologists established that a specific series of cell shape changes is associated with various stages of the process. “Until now there was no quantitative mechanical understanding of whether those changes were sufficient to account for the observed embryo shapes, and existing studies by conventional microscopy were limited to two-dimensional sections and analyses of chemically fixed embryos, rendering comparisons with theory on the dynamics difficult,” said Professor Raymond E. Goldstein of the Department of Applied Mathematics and Theoretical Physics, who led the research.
The interdisciplinary group of Cambridge scientists obtained the first three-dimensional visualisations of Volvox inversion and developed a first mathematical model that explains how cell shape changes drive the process of inversion.
Their time-lapse recordings show that during inversion one hemisphere of the embryos shrinks while the other hemisphere stretches out. While previous studies on fixed embryos have also observed this phenomenon, the question was if these changes are caused by forces produced within the invaginating region, or from elsewhere in the embryo.
Through mathematical modelling, the researchers found that only if there is active contraction of one hemisphere and active expansion of the other does the model yield the observed ‘mushroom’ shape of an inverting Volvox globator embryo.
“It’s exciting to be able to finally visualise this intriguing process in 3D,” said Dr Stephanie Höhn, the paper’s lead author. “This simple organism may provide ground-breaking information to help us understand similar processes in many different types of animals.”
These results imply that any cell shape changes happening away from the invagination region seem to be due to active forces intrinsic to the cell, rather than through passive deformations. Since analyses in animal model organisms mostly concentrate on cell shape changes that happen within an invaginating region, the model could be used to make those analyses far more accurate.
“The power of this mathematical model is that we can identify which cell deformations are needed to cause the embryo movements that we observe in nature,” said Dr Aurelia Honerkamp-Smith, one of the study’s co-authors.
The experimental and theoretical methods demonstrated in this study will be expanded to understand not only the peculiar inversion process but many mysteries concerning morphogenesis. The mathematical model may have applications in a multitude of such topological problems, such as the process of neurulation that leads to the enclosure of the tissue that eventually becomes the spinal cord.