Researchers have linked a species of intestinal bacteria known as Prevotella copri to the onset of rheumatoid arthritis, the first demonstration in humans that the chronic inflammatory joint disease may be mediated in part by specific intestinal bacteria. The new findings by laboratory scientists and clinical researchers in rheumatology atNYU School of Medicine add to the growing evidence that the trillions of microbes in our body play an important role in regulating our health.
Using sophisticated DNA analysis to compare gut bacteria from fecal samples of patients with rheumatoid arthritis and healthy individuals, the researchers found that P. copri was more abundant in patients newly diagnosed with rheumatoid arthritis than in healthy individuals or patients with chronic, treated rheumatoid arthritis. Moreover, the overgrowth of P. copri was associated with fewer beneficial gut bacteria belonging to the genera Bacteroides.
“Studies in rodent models have clearly shown that the intestinal microbiota contribute significantly to the causation of systemic autoimmune diseases,” says Dan R. Littman, MD, PhD, the Helen L. and Martin S. Kimmel Professor of Pathology and Microbiology and a Howard Hughes Medical Institute investigator.
“Our own results in mouse studies encouraged us to take a closer look at patients with rheumatoid arthritis, and we found this remarkable and surprising association,” says Dr. Littman, whose basic science laboratory at NYU School of Medicine’s Skirball Institute of Biomolecular Medicine collaborated with clinical investigators led bySteven Abramson, MD, senior vice president and vice dean for education, faculty, and academic affairs; the Frederick H. King Professor of Internal Medicine; chair of the Department of Medicine; and professor of medicine and pathology at NYU School of Medicine.
“At this stage, however, we cannot conclude that there is a causal link between the abundance of P. copri and the onset of rheumatoid arthritis,” Dr. Littman says. “We are developing new tools that will hopefully allow us to ask if this is indeed the case.”
The new findings, reported today in the open-access journal eLife, were inspired by previous research in Dr. Littman’s laboratory, collaborating with Harvard Medical School investigators, using mice genetically predisposed to rheumatoid arthritis, which resist the disease if kept in sterile environments, but show signs of joint inflammation when exposed to otherwise benign gut bacteria known as segmented filamentous bacteria.
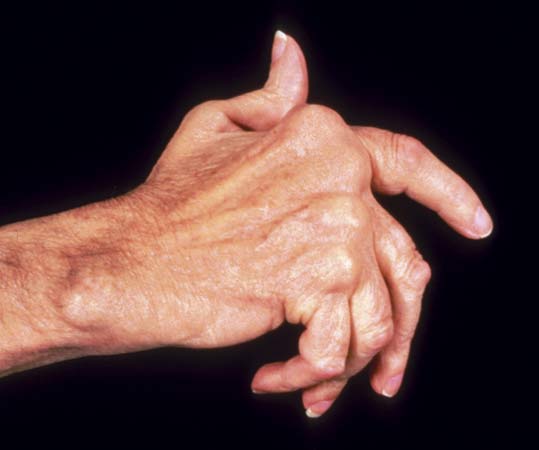
Rheumatoid arthritis, an autoimmune disease that attacks joint tissue and causes painful, often debilitating stiffness and swelling, affects 1.3 million Americans. It strikes twice as many women as men and its cause remains unknown although genetic and environmental factors are thought to play a role.
The human gut is home to hundreds of species of beneficial bacteria, including P. copri, which ferment undigested carbohydrates to fuel the body and keep harmful bacteria in check. The immune system, primed to attack foreign microbes, possesses the extraordinary ability to distinguish benign or beneficial bacteria from pathogenic bacteria. This ability may be compromised, however, when the gut’s microbial ecosystem is thrown off balance.
“Expansion of P. copri in the intestinal microbiota exacerbates colonic inflammation in mouse models and may offer insight into the systemic autoimmune response seen in rheumatoid arthritis,” says Randy S. Longman, MD, PhD, a post-doctoral fellow in Dr. Littman’s laboratory and a gastroenterologist at Weill-Cornell, and an author on the new study. Exactly how this expansion relates to disease remains unclear even in animal models, he says.
Why P. copri growth seems to take off in newly diagnosed patients with rheumatoid arthritis is also unclear, the researchers say. Both environmental influences, such as diet and genetic factors can shift bacterial populations within the gut, which may set off a systemic autoimmune attack. Adding to the mystery, P. copri extracted from stool samples of newly diagnosed patients appears genetically distinct from P. copri found in healthy individuals, the researchers found.
To determine if particular bacterial species correlate with rheumatoid arthritis, the researchers sequenced the so-called 16S gene on 44 fecal DNA samples from newly diagnosed patients with rheumatoid arthritis prior to immune-suppressive treatment; 26 samples from patients with chronic, treated rheumatoid arthritis; 16 samples from patients with psoriatic arthritis (characterized by red, flaky skin in conjunction with joint inflammation); and 28 samples from healthy individuals.
Seventy-five percent of stool samples from patients newly diagnosed with rheumatoid arthritis carried P. copricompared to 21.4% of samples from healthy individuals; 11.5% from chronic, treated patients; and 37.5% from patients with psoriatic arthritis.
Rheumatoid arthritis is treated with an assortment of medications, including antibiotics, anti-inflammatory drugs like steroids, and immunosuppressive therapies that tame immune reactions. Little is understood about how these medications affect gut bacteria. This latest research offers an important clue, showing that treated patients with chronic rheumatoid arthritis carry smaller populations of P. copri. “It could be that certain treatments help stabilize the balance of bacteria in the gut,” says Jose U. Scher, MD, director of the Microbiome Center for Rheumatology and Autoimmunity at NYU Langone Medical Center’s Hospital for Joint Diseases, and an author on the new study. “Or it could be that certain gut bacteria favor inflammation.”
The researchers plan to validate their results in regions beyond New York, since gut flora can vary across geographical regions, and investigate whether the gut flora can be used as a biological marker to guide treatment. “We want to know if people with certain populations of gut bacteria respond better to certain treatment than others,” says Dr. Scher. Finally, they hope to study people before they develop rheumatoid arthritis to see whether overgrowth of P. copri is a cause or result of autoimmune attacks.
In addition to researchers from the NYU School of Medicine, investigators from Memorial Sloan Kettering Cancer Center and from the Harvard School of Public Health contributed to the study. Funding for this research comes from the National Institutes of Health, the Howard Hughes Medical Institute, and the American Gastroenterological Association.


Great article . Thanks for sharing.