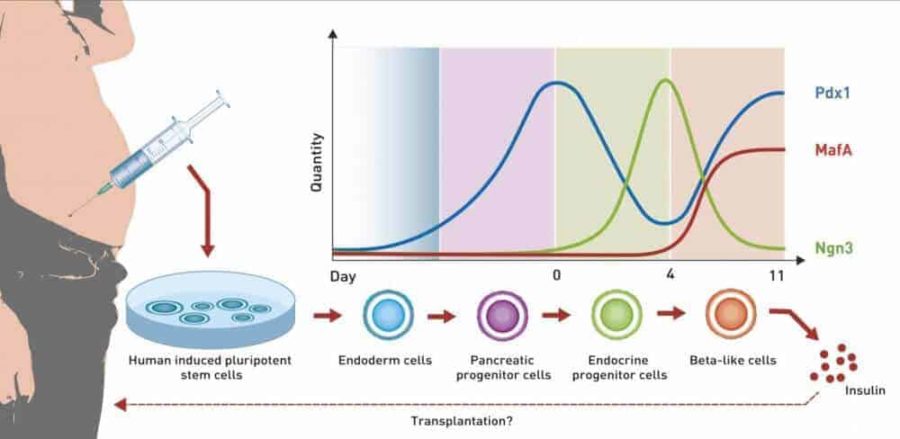
Researchers led by Martin Fussenegger, Professor of Biotechnology and Bioengineering at ETH Zurich’s Department of Biosystems Science and Engineering in Basel, have performed a feat that many specialists had until now held to be impossible: they have extracted stem cells from a 50-year-old test subject’s fatty tissue and applied genetic reprogramming to make them mature into functional beta cells.
In the presence of glucose, the beta cells generated using this “genetic software” produce the hormone insulin – just like natural beta cells, which are found in the pancreas. The researchers reported this in the journal Nature Communications.
Maturation dynamic reproduced
The Basel-based researchers took the stem cells and added a highly complex synthetic network of genes – the genetic software. They designed this network to precisely recreate the key growth factors involved in this maturation process.
Central to the process are the growth factors Ngn3, Pdx1 and MafA. Concentrations of these factors change during the differentiation process. For instance, MafA is not present at the start of maturation. Only on day four, in the final maturation step, does it appear, its concentration rising steeply and then remaining at a high level. The changes in concentration of Ngn3 and Pdx1, however, are very complex: while the concentration of Ngn3 rises and then falls again, the level of Pdx1 rises at the beginning and towards the end of maturation.
Fussenegger stresses that it is essential to reproduce these natural processes as closely as possible in order to produce functioning beta cells: “The timing and the quantities of these growth factors are extremely important.”
New beta cells respond to glucose
In Fussenegger’s opinion, it is a real breakthrough that a synthetic gene network has been successfully used to achieve genetic reprogramming that delivers beta cells. Until now, scientists have controlled such stem cell differentiation processes by adding various chemicals and proteins using pipettes.
“It’s not only really hard to add just the right quantities of these components at just the right time, it’s also inefficient and impossible to scale up,” Fussenegger says. In contrast, the new process can successfully transform three out of four adipose stem cells into beta cells.
These beta cells not only look very similar to their natural counterparts – both kinds contain dark spots known as granules, which store insulin. The artificial beta cells also function in a very similar way. “At the present time, the quantities of insulin they secrete are not as great as with natural beta cells,” he admits.
But the key point is that the researchers have for the first time succeeded in reproducing the entire natural process chain, from stem cell to differentiated beta cell.
Implants of endogenous cells
In future, the Basel-based ETH researchers’ new technique might make it possible to implant new functional beta cells in diabetes sufferers that are made from their own adipose tissue.
While beta cells have been transplanted in the past, this has always required subsequent suppression of the recipient’s immune system – as with any transplant of donor organs or tissue. “With our beta cells, there would likely be no need for this action, since we can make them using endogenous cell material taken from the patient’s own body,” Fussenegger says, adding: “This is why our work is of such interest in the treatment of diabetes.”
Complete maturation in the petri dish
To date, the ETH researchers have merely cultured their beta cells; they have yet to implant them in a diabetes sufferer. This is because they first wanted to test whether stem cells could be fully differentiated from start to finish using genetic programming.
Fussenegger is convinced that this new method could also be used to produce other cells. Stem cells taken from adipose tissue could be differentiated into various cell types, he says – “And most people have an overabundance of fat from which these stem cells can be harvested.”