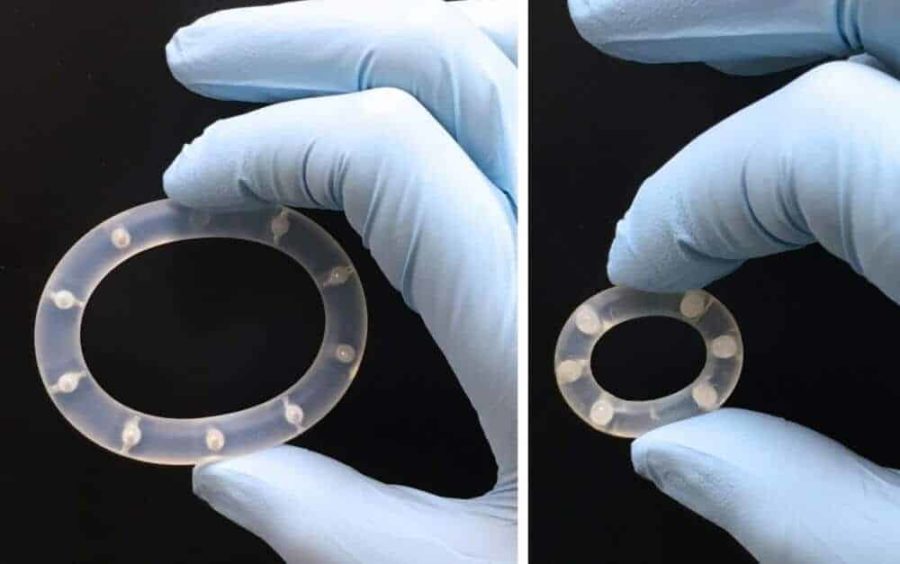
Researchers are edging ever closer to discovering the perfect combination of drugs and drug delivery system that will stop the sexual transmission of HIV. Findings published last week in the journal PLOS ONE confirm that researchers from the Oak Crest Institute of Science, located in Monrovia, CA, have demonstrated for the first time that two powerful antiretroviral (AVR) drugs can provide complete protection against HIV when delivered topically by a sustained release intravaginal ring (IVR) device.
Results of the study, Topical Delivery of Tenofovir Disoproxil Fumarate and Emtricitabine from Pod-Intravaginal Rings Protect Macaques from Multiple SHIV Exposures, confirm that the combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) delivered at independently controlled rates via the pod-IVR were successful at preventing SHIV162p3 infection in a rigorous, repeat low-dose vaginal exposure model using normally cycling female pigtailed macaques.
“We are extremely pleased with the results of this study,” says Dr. Marc M. Baum, president and senior faculty at Oak Crest. “The observed protection in macaques indicates the significant potential for the TDF-FTC drug combination delivered via the pod-IVR to successfully prevent sexual HIV infection in humans.”
Throughout the six-month study, conducted at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, six macaques received pod-IVRs containing 65 mg of TDF and 68 mg of FTC every two weeks. One week after the first pod-IVR insertion, the macaques received the first of 16 vaginal exposures to 50 TCID50 of SHIV162p3, a chimeric SIV/HIV virus that infects macaques. The rings were exchanged every two weeks (three days prior to the next weekly virus exposure) corresponding to a total of eight ring changes during the 18-week period, with the final ring removal at week 18. Complete protection was achieved in all (six of six) animals with TDF-FTC pod-IVRs compared to 0 of 9 control animals without pod-IVRs over four months of weekly virus exposures that spanned several menstrual cycles.
Research on the pigtailed macaque model is particularly relevant because of its similarities with the human menstrual cycle, vaginal architecture and vaginal microbiome, and the ability to conduct efficacy studies with simian-human immunodeficiency virus. “This model has been used extensively to evaluate several lead candidates for preexposure prophylaxis (PrEP) against HIV,” says Dr. Baum. “We are able to critically evaluate the pharmacokinetics, safety, and efficacy of PrEP candidates in ways that are not feasible in humans, thereby allowing us to make informed decisions on candidates in the PrEP pipeline prior to initiation of costly clinical trials.”
“Up to this point, topical preexposure prophylaxis (PrEP) against HIV has been marginally successful in recent clinical trials with low adherence rates being a primary factor for failure,” states Dr. Baum. “Our hypothesis has been that controlled, sustained release of ARV drug combinations could overcome these low adherence rates if the product itself could remain protective for extended periods of time. This study has shown that the drug combination and the versatile drug delivery system that we have designed can indeed remain protective for over four and a half months.”
The development of this technique of drug delivery eliminates many of the challenges related to oral administration of the desired drugs. Issues such as adherence to a regular dosing schedule are eliminated by the continuous release of the drugs into the vaginal tissues. The steady release of drugs into the system also decreases side effects as the medications are absorbed into the system in a steady dose throughout the 28-day cycle.
“In contrast to daily therapies, sustained release approaches to drug delivery have special appeal for use in the developing world: they are less expensive on a per-patient, per-day basis, they require less infrastructure to provide to the community, and they can be more effective,” adds Dr. Baum.
Since the beginning of the AIDS epidemic, almost 78 million people have become infected with HIV. More than 50% of those living with HIV are women, with more than 350,000 new infections in young women (15-24 years old) estimated to occur each year. Most of these infections were acquired through heterosexual intercourse. With an HIV vaccine still years away, the use of AVRs in preexposure prophylaxis (PrEP) is a promising biomedical intervention to prevent new infections. The pod-IVR is especially well suited for use by women in developing nations where a highly effective, low cost form of HIV prevention is in high demand.
According to Dr. Baum, the choice of drugs available for HIV PrEP has expanded greatly in recent years, and many ARVs that were historically used exclusively for HIV treatment have now been added to the PrEP pipeline. ARV drugs from multiple classes are currently being evaluated in preclinical models to assess their effectiveness as a PrEP agent.
Currently, the only FDA-approved ARV drug for HIV PrEP, is the oral combination of TDF and FTC. “The novel and versatile drug delivery system that our team is developing has proven that it has the capacity to deliver and maintain protective levels of multiple drugs,” states Dr. Baum. “Protection against (S)HIV virus that was observed in this study warrants clinical evaluation of the pod-IVR design and the sustained delivery of a variety of AVRs to find the best combination possible.”