For the first time, scientists have generated endometrial cells from induced pluripotent stem cells, according to a recent Northwestern Medicine study published in Stem Cell Reports.
This new technique will allow scientists to explore new treatments for a variety of uterine conditions, according to Serdar Bulun, MD, chair and John J. Sciarra Professor of Obstetrics and Gynecology and senior author of the study.
The use of induced pluripotent stem cells (iPSC) has become widespread over the past several years; as an alternative to highly-regulated embryonic stem cells, iPSC are a cellular blank slate that scientists can prod and nudge into becoming nearly any type of cell in the body. Using specific hormones and growth factors, scientists can encourage these cells to transform — or differentiate — into motor neurons, lung cells, vascular cells and more.
Up until now, however, scientists have yet to develop a reliable technique to create endometrial cells, which line the uterus and are important for fertility and the menstrual cycle.
“We used what was available in the literature to make iPSC into intermediate mesodermal cells,” said Bulun, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “From that point forward, instead of moving them in the direction of kidney cells, we wanted to move them in the direction of uterine cells.”To achieve this goal, Bulun and his collaborators didn’t start from scratch; other scientists had previously created kidney cells from iPSC, and kidney and endometrial cells share a common ancestor: intermediate mesoderm cells.
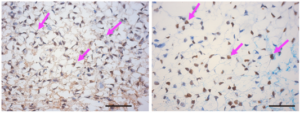
One key distinction of endometrial cells is their response to progesterone, a hormone that plays key roles in the menstrual cycle and embryo implantation. Previous studies had revealed a pathway called WNT/CTNNB1 was important for uterine development, so the Northwestern scientists activated the pathway in the stem cells.
By activating WNT, and applying other growth factors, the scientists guided these cells from an undifferentiated stem cell to an intermediate mesoderm and eventually to an endometrial cell.
“We were looking for progesterone receptors,” Bulun said. “When we saw lots of those little receptors, we knew we had done it.”
These cells could help scientists devise treatments for a variety of uterine diseases, many of which, including endometriosis and endometrial cancer, are thought to be at least partially caused by disordered interactions with progesterone.
In endometriosis, the cells are not responding because they have decreased levels of progesterone receptors, according to Bulun.
“This technique may be a way to reprogram a patient’s cells so that they respond to progesterone properly,” Bulun said. “These new cells and their derivatives could possibly be used to regenerate the entire uterus.”
Endometrial cancer could conceivably be treated in a similar way, Bulun said.
“All of these things can be explored, now that we have this technology,” he said.
Bulun’s next steps are to explore just how close these iSPC-derived endometrial cells are to the real thing, and to work with clinicians and surgeons to develop delivery methods for the cells. Bulun said he is particularly looking forward to seeing other labs use this technique to create endometrial cells of their own.
“It’s very rewarding, when other people reproduce your work,” Bulun said. “It’s exactly why we did this.”
Other Northwestern authors include Bulun laboratory members John Coon V, laboratory manager, and Kaoru Miyazaki, MD, PhD, postdoctoral fellow and first author of the study. Yuichi Furukawa and Bahar Yilmaz were also co-authors.
The study was supported by funding from National Institutes of Health grants R37-HD36891, the Uehara Memorial Foundation and the Kanazawa Medical Research Foundation.