More than two-thirds of people with an advanced form of a rare skin cancer are on track to survive at least two years after starting an immunotherapy drug on a landmark clinical trial, researchers reported Wednesday in the Journal of Clinical Oncology.
That’s a profound shift for this deadly cancer, Merkel cell carcinoma, or MCC. Just a few years ago, patients with advanced MCC would invariably receive chemotherapy, the previous standard of care. As many as 75 percent would die within that timeframe, previous studies have shown.
The investigators are “thrilled” that so many of the trial participants are doing well, compared with what historical data would predict, said Martin “Mac” Cheever of Fred Hutchinson Cancer Research Center, one of the leaders of this first-of-its-kind study.
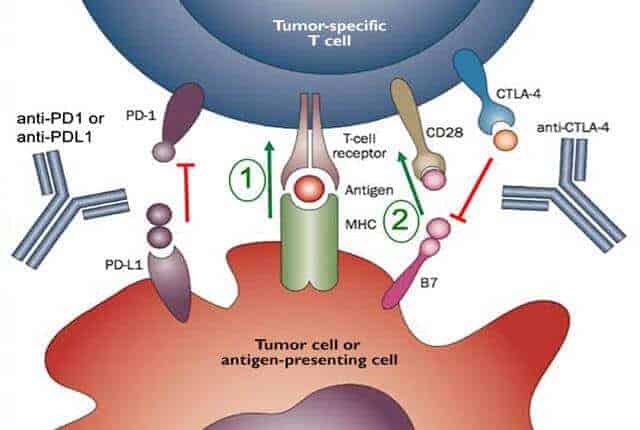
The multisite trial tested an immune-boosting drug called pembrolizumab (Keytruda), also known as a checkpoint inhibitor because it blocks a checkpoint that keep the immune system from mounting a cancer-killing response.
Fifty patients were enrolled in the (not randomized) trial. After receiving pembrolizumab, 28 saw their cancers shrink or even disappear — an unusually high rate of response for a solid-tumor cancer, Cheever said. For almost all of these patients, the results were sustained two years after their first dose of the drug, the researchers reported. Based on their results to date, the research team calculated a two-year survival rate of more than 68 percent.
The drug is not without risks. In their most recent report, the scientists wrote that the trial drug likely contributed to severe side effects in 14 of the 50 patients, including one death.
The U.S. Food and Drug Administration approved pembrolizumab for MCC in December 2018, based on the strength of its results. But the drug has been standard care for patients with MCC since 2015, when experts rewrote medical guidelines after the trial’s preliminary results emerged.
As noteworthy as the findings have been, Cheever said, “it’s the beginning, not the end.”
“We’re also coming up with a lot of information that will help design future trials for this disease,” he said. At least four pharmaceutical companies are developing drugs for this once-neglected disease, he added.
The trial was conducted at 13 sites nationwide through the Cancer Immunotherapy Trials Network, a federally funded research group headquartered at Fred Hutch. The trial was initiated by Cheever and principal investigator Paul Nghiem, head of dermatology at the University of Washington School of Medicine.
The trial was funded by the National Institutes of Health, private donors, the Prostate Cancer Foundation and Merck.