Diabetes mellitus is associated with various health problems including decline in skeletal muscle mass. A research group led by Professor Wataru Ogawa at the Kobe University Graduate School of Medicine revealed that elevation of blood sugar levels leads to muscle atrophy and that two proteins, WWP1 and KLF15, play key roles in this phenomenon. These findings were published on February 21 in the online edition of JCI Insight.
Muscle mass decline associated with ageing impairs our physical activity, making us susceptible to a variety of health problems and thus leading to shortened lifespans. Age-dependent muscle mass decline and the consequent impairment of physical activity is known as “sarcopenia”, a serious health burden in aging societies.
We already knew that patients with diabetes mellitus are prone to muscle loss as they age, but an underlining mechanism for this phenomenon remains unclear. Diabetes mellitus is a disease caused by insufficient action of the hormone insulin. Insulin not only lowers blood sugar levels, but promotes the growth and proliferation of cells; insufficient action of insulin has been thought to result in the suppression of growth and proliferation of muscle cells, which in turn contribute to the decline in skeletal muscle mass.
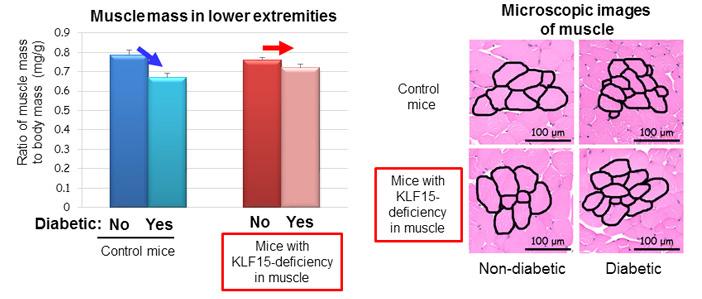
Professor Ogawa’s research team made the surprising discovery that a rise in blood sugar levels triggers the decline in muscle mass, and uncovered the important roles of two proteins in this phenomenon. They found that the abundance of transcription factor KLF15 increased in skeletal muscle of diabetic mice, and mice that lack KLF15 specifically in muscle were resistant to diabetes-induced skeletal muscle mass decline (Figure 1). These results indicate that diabetes-induced muscle loss is attributable to increased amounts of KLF15.
The team investigated the mechanism for how the abundance of KLF15 is increased in skeletal muscle of diabetic mice. They found that elevation of blood sugar levels slows down the degradation of KLF15 protein, which leads to an increased amount of this protein. Professor Ogawa’s team also discovered that a protein called WWP1 plays a key role in regulating the degradation of KLF15 protein.
WWP1 is a member of proteins called ubiquitin ligase. When a small protein called “ubiquitin” binds to other proteins, the degradation of the ubiquitin-bound proteins is accelerated. Under normal conditions, WWP1 promotes the degradation of KLF15 protein by binding ubiquitins to KLF15, keeping cellular KLF15 abundance low. When blood sugar levels rise, the amount of WWP1 decreases, which in turn decelerates the degradation of KLF15 and thus the increase in the cellular abundance of KLF15 (Figure 2).
This study uncovered for the first time that elevation of blood sugar levels triggers muscle mass decline, and that the two proteins WWP1 and KLF15 contribute to diabetes-induced muscle mass decline.
As well as diabetes mellitus, other conditions such as physical inactivity or ageing result in muscle mass loss. The proteins KLF15 and WWP, which have been shown to contribute to diabetes-induced muscle mass loss, may also be related to other causes of muscle loss. Currently, no drug is available for the treatment of muscle loss. Professor Ogawa comments: “If we develop a drug that strengthens the function of WWP1 or weakens the function of KLF15, it would lead to a groundbreaking new treatment”.