Scientists from the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg and from the German Cancer Research Center (DKFZ) have been able to rejuvenate stem cells in the brain of aging mice. The revitalised stem cells improve the regeneration of injured or diseased areas in the brain of old mice. The researchers expect that their approach will provide fresh impetus in regenerative medicine and facilitate the development of stem cell therapies.
Their results were published today in the journal Cell (DOI: https:/
In order to create as accurate as possible computational models of stem cell behaviour, the LCSB’s Computational Biology Group led by Prof. Antonio del Sol applied a novel approach. “Stem cells live in a niche where they constantly interact with other cells and extra-cellular components. It is extremely difficult to model such a plethora of complex molecular interactions on the computer. So we shifted perspective. We stopped thinking about what external factors were affecting the stem cells, and started thinking about what the internal state of a stem cell would be like in its precisely defined niche.”
The novel approach led to in a new computational model developed by Dr. Srikanth Ravichandran of the Computational Biology Group: “Our model can determine which proteins are responsible for the functional state of a given stem cell in its niche – meaning whether it will divide or remain in a state of quiescence. Our model relies on the information about which genes are being transcribed. Modern cell biology technologies enable profiling of gene expression at single cell resolution.”
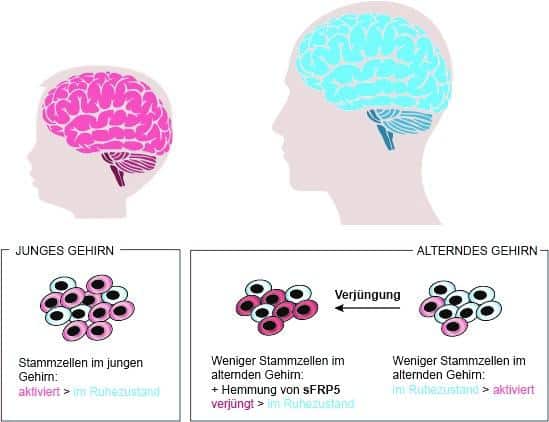
It was previously unknown why most of the stem cells in the brain of old mice remain in a state of quiescence. From their computational model, the researchers at the LCSB identified a molecule called sFRP5 that keeps the neuronal stem cells inactive in old mice, and prevents proliferation by blocking the Wnt pathway crucial for cell differentiation.
A rejuvenation for cells
Then the long-standing expertise in neural stem cells of the collaborators at the German Cancer Research Center (DKFZ) came in: Studying stem cells first in a dish and then later directly in mice, they could experimentally validate the computational prediction. When neutralising the action of sFRP5, the quiescent stem cells did indeed start proliferating more actively. Thus, they were available again to be recruited for the regeneration processes in the aging brain. “With the deactivation of sFRP5, the cells undergo a kind of rejuvenation,” del Sol says: “As a result, the ratio of active to dormant stem cells in the brain of old mice becomes almost as favourable as in young animals.”
“Our results constitute an important step towards the implementation of stem cell-based therapies, for instance for neurodegenerative diseases,” Antonio del Sol says. “We were able to show that, with computational models, it is possible to identify the essential features that are characteristic of a specific state of stem cells.” This approach is not limited to studying the brain. It can also be used to model stem cells of other organs in the body. “The hope is that this will open avenues for regenerative medicine,” says del Sol.