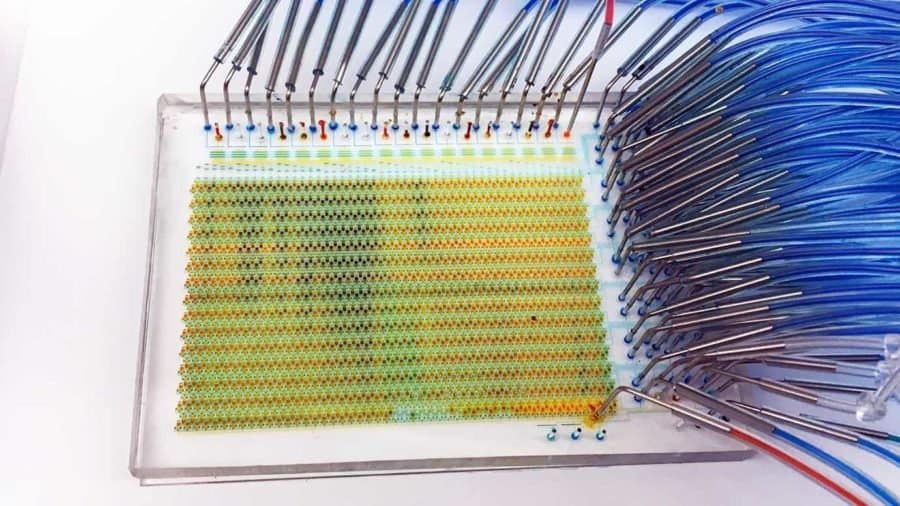
Researchers with the Institute for Molecular Engineering at the University of Chicago have developed a new “lab-on-a-chip” that can examine thousands of individual live cells over a weeklong period, performing experiments that would take more than 1 million steps in a laboratory.
The credit-card-sized, microfluidic device not only saves time and money, but also offers a new glimpse into how single stem cells react to different molecules and environments.
When researchers examined neural stem cells on the device and analyzed the data, they found several new rules that determine the timing and signaling sequences needed to cause the cells to differentiate or renew themselves. The finding could have implications in understanding brain development or in treating patients with immunotherapy.
“We wanted to develop a microfluidic device that could sort, image and culture single cells in an automated, high-throughput way,” said Assoc. Prof. Savas Tay, lead author of the research, published April 3 in the journal Science Advances. “We achieved that, and now we have an understanding of how stem cells make decisions. That’s pretty exciting.”
Developing a new way to study cells
Cells within our body are constantly responding to different signals and changes in the environment. In stem cells, for example, signals received at different points in time determine how the cell chooses what kind of cell it will develop into. One signal might cause a stem cell to differentiate into another cell, while another signal might cause it to maintain its form.
Researchers currently have no way of studying these signal molecules on individual cells inside the body. Such analysis can be done in a lab with expensive, time-consuming experiments, but they ultimately cannot test all possible outcomes.
Microfluidic devices, which have tiny chambers, tunnels and valves, have offered researchers a faster, automated process for studying these reactions in cells. But these devices have offered a limited number of chambers—meaning researchers could only test a certain amount of conditions with each cell—and could not keep the cells alive long enough to study them over a long period of time.
Finding a way to keep finicky cells alive
Tay and his collaborators set out to change that. They designed a microfluidic device that has 1,500 automated chambers—much higher than similar devices, which have less than 100. The device can also conduct several tasks—like cell stimulation, culturing, imaging and sorting—that were previously relegated to separate devices. It can culture cells in different modes—meaning it can examine different types of cells at the same time.
Finally, the device also can keep cells alive for much longer, thanks to a new technique of diffusing media into a cell culture. Normally, to keep cells alive, researchers must change the media they are kept in every few hours. This change shocks the cells, and after several shocks, the cells can die. The researchers’ new technique diffuses the media into the cell chamber, a gentler process that does not shock the cells.
In the first experiment with the device, the researchers studied how different signaling molecules affected the outcome of mouse neural stem cells. Such experiments create millions of data points, so Tay collaborated with Andrey Rzhetsky, UChicago professor in medicine and genetics, to conduct machine-learning analyses on the large dataset.
They found that certain combinations of signals synergize and cause the cells to differentiate, while other molecules shut down those processes. The timing of these signals is also crucial. If a molecule is delivered at the right time, the researchers found, it can change the course of stem cells, from differentiation to self-renewal.
“There are certain orders of signals that are highly optimal, and the exact timing of signals matters,” Tay said. “There hasn’t been a way to dynamically monitor these cells before, so finding and understanding these principles is exciting.”
Next, the researchers hope to use the device to study organoids, tissue cultures derived from stem cells that organize themselves like tiny organs.
Ultimately, a device like this could be used in fields like immunotherapy, where a patient’s own immune system is stimulated to help fight disease. A patient’s stem cells could be removed, placed into the device and be given the right combination of molecules to develop them into a certain lineage, then be placed back in the body.
“We want to be able to use this device for all kinds of problems in cell biology,” Tay said.
Other authors on the paper include Ce Zhang and Hsiung-Lin Tu, former postdoctoral fellows in Tay’s lab; Gengjie Jia, a postdoc in the Rzhetsky lab; and Verdon Taylor and Tanzila Mukhtar of the University of Basel.
Citation: “Ultra-multiplexed analysis of single-cell dynamics reveals logic rules in differentiation.” Zhang et al, Science Advances, doi: 10.1126/sciadv.aav7959