The heart of humans and mice does not completely recover after a heart attack. It now turns out that cells close to area of the heart attack respond to the damage resulting from the heart attack, and that this response is important for survival. This was discovered by researchers from the groups of Jeroen Bakkers (Hubrecht Institute) and Vincent Christoffels (Amsterdam UMC). Additional research on these cells, and on similar cells in animals in which the heart does completely recover after a heart attack, may lead to new treatments for patients with heart damage in the future. The results of this research were published in the scientific journal Circulation.
Heart attack
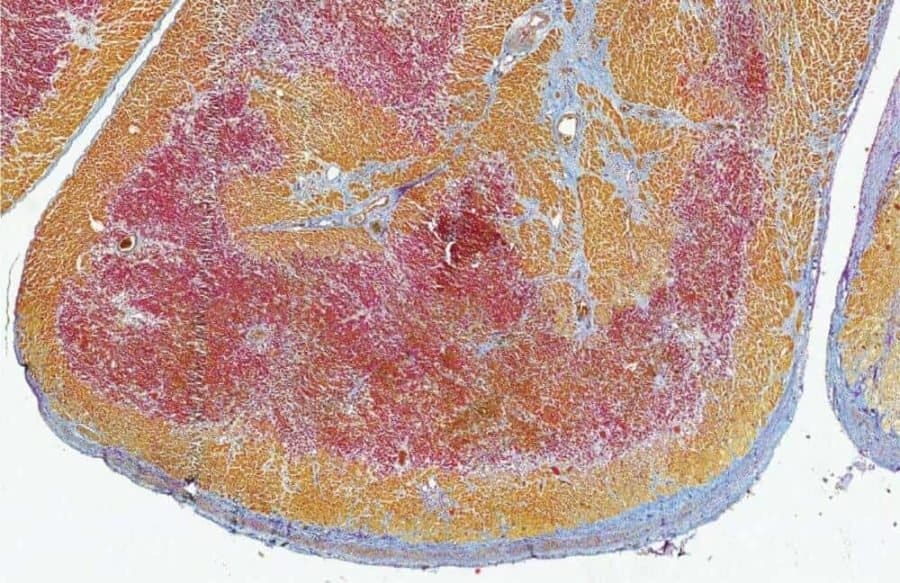
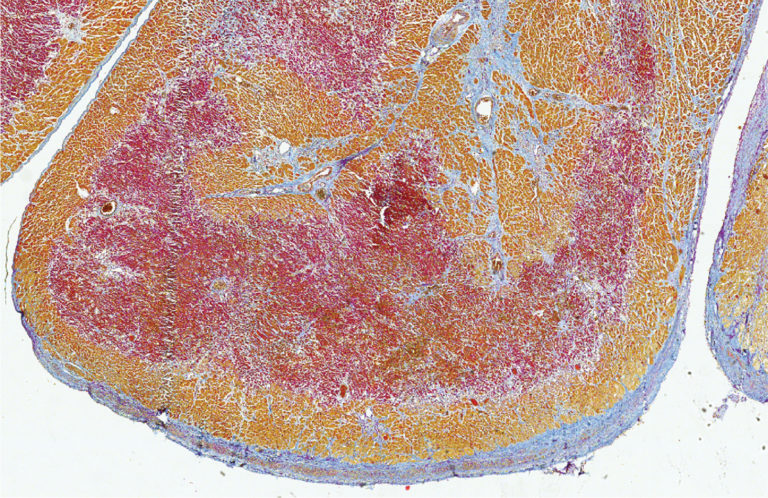
Cardiovascular diseases remain the main cause of death in the Western world. After a heart attack, scar tissue replaces the lost heart muscle. Because of this, the damage to the heart is permanent and patients are considered to have chronic heart disease. In some animals however, such as the zebrafish, the heart does fully recover after a heart attack. Researchers have already discovered previously that this recovery happens in the healthy heart tissue directly adjacent to the damaged area of the heart. This area is called the border zone. Studying this border zone in different species can teach us more about the response of the heart to a heart attack, so that we may eventually induce this response in humans to recover the lost heart muscle after a heart attack.
Important gene
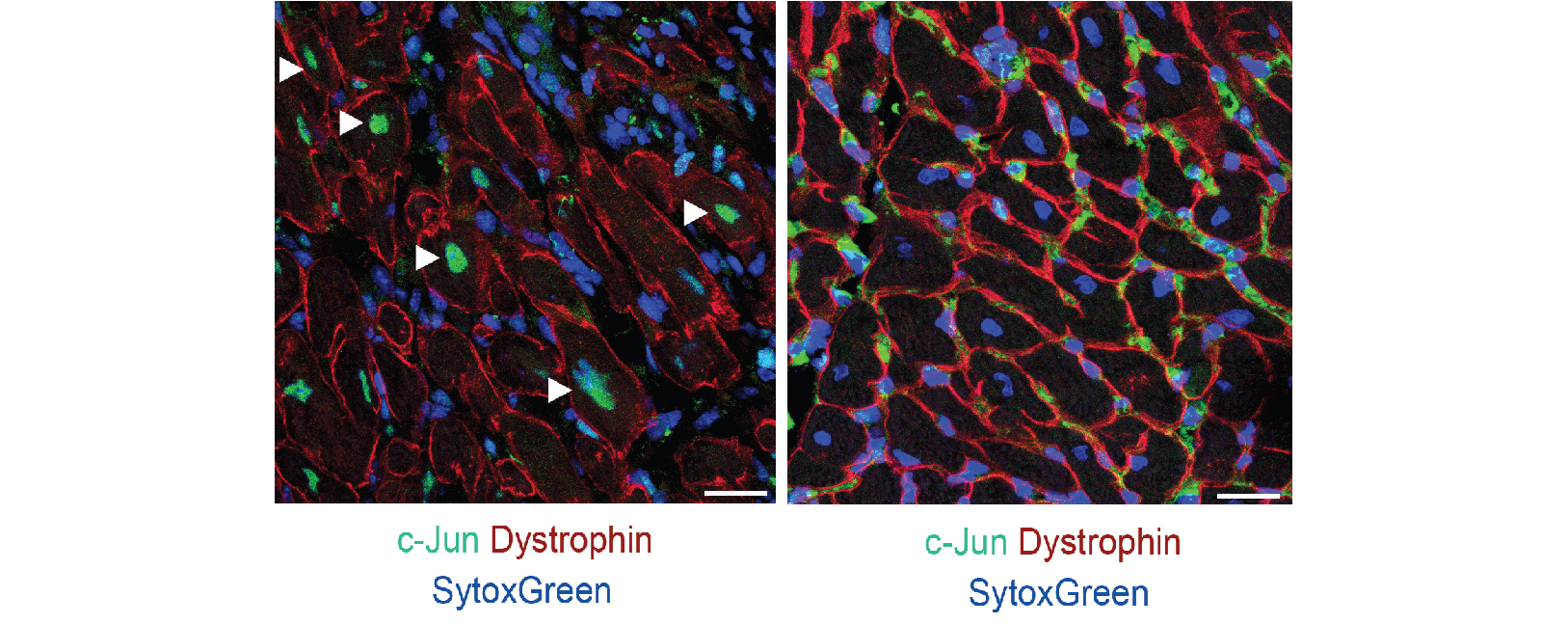
Researchers have now discovered that the mouse heart, which is permanently damaged after a heart attack just like the human heart, also develops a border zone. “In the border zone cells, the gene program that is normally active in the heart muscle cells was replaced by a gene program that helps the cells deal with the heart damage,” says Karel van Duivenboden, researcher in the group of Vincent Christoffels (Amsterdam UMC). The researchers discovered that this program is incredibly important for surviving a heart attack. When they turned off one of the genes in this program, called NPPB, in mice with a heart attack, the chance of survival became much smaller.
Humans
By studying heart tissue of patients that had a heart attack, the researchers also identified a border zone in humans. This border zone turns out to respond in a similar way to a heart attack as the border zone in mice. However, the human border zone turned out to be located in a different area than doctors had thought until now.
Dream for the future
“Now that we know that humans also develop a border zone after a heart attack, we can start to investigate why the heart of humans and mice does not recover after a heart attack, while the hearts of some other animals do,” explains Dennis de Bakker, researcher in the group of Jeroen Bakkers (Hubrecht Institute). We may eventually be able to activate cells in the border zone to start growing back the lost heart tissue, in order to restore the heart after a heart attack. “For now, however, this remains a dream for the future,” according to De Bakker.
Publication
Conserved NPPB+ border zone switches from MEF2 to AP-1 driven gene program. Karel van Duijvenboden*, Dennis E.M. de Bakker*, Joyce C.K. Man*, Rob Janssen*, Marie Günthel*, Matthew C. Hill, Ingeborg B. Hooijkaas, Ingeborg van der Made, Petra H. van der Kraak, Aryan Vink, Esther E. Creemers, James F. Martin, Phil Barnett, Jeroen Bakkers#, Vincent M. Christoffels#. Circulation 2019.
* equal contribution, # shared last author