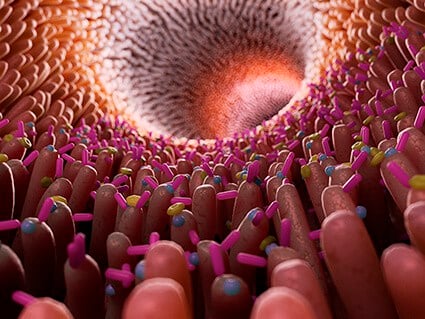
It’s now accepted that gut-barrier dysfunction and gut-derived chronic inflammation play a role in human aging, but how that process is regulated is still largely a mystery. A team led by Richard Hodin, MD, chief of the Division of General and Gastrointestinal Surgery at Massachusetts General Hospital (MGH) has uncovered an important piece of the puzzle which is described in the journal JCI Insight.
Studying mice and fruitflies, researchers found that the enzyme intestinal alkaline phosphotase (IAP) helped prevent intestinal permeability and gut-derived systemic inflammation, resulting in less frailty and extended life span. “Oral IAP supplementation in older mice significantly preserved gut barrier function and was associated with preserving the homeostasis of the gut microbiota during aging,” said Hodin. “In other words, the enzyme maintained the composition of the gut bacteria and controlled the low-grade chronic inflammation that can happen with aging.”
Because the scientists were using animal models, they were able to test blood from the portal venous system, which goes from the GI tract into the liver and then on through the rest of the body. “This gave us a more direct measure of what’s passing across the gut barrier than blood from a human arm would,” said Hodin, who began studying the function of IAP over a decade ago after connecting the dots in a couple of obscure papers suggesting the enzyme blocks an endotoxin called LPS. “I was studying IAP for other reasons, not knowing anything about its function,” he said. “No one really did.”
Because IAP is a naturally occurring enzyme that almost entirely remains in the gut rather than traveling throughout the system, Hodin believes it should prove nontoxic to humans, and those who are found have low levels, especially as they age, will simply be able to supplement. “Because IAP confers anti-inflammatory properties systemically,” Hodin said, “it could have implications not only for inflammatory bowel diseases like Crohn’s and ulcerative colitis, but also for other human conditions that we now know are linked to the gut and inflammation, such as obesity and diabetes.” The team is already at work on the next steps toward getting FDA approval for a supplement. The study’s co-authors are Florian Kühn, of the Department of Surgery at MGH and the University Hospital of Munich (LMU); Fatemeh Adiliaghdam, Paul M. Cavallaro, Sulaiman R. Hamarneh, Amy Tsurumi, Alexander R. Munoz, Yashoda Dhole, Juan M. Ramirez, Enyu Liu, Robin Vasan, Yang Liu, Shsan Samarbafzadek, Rocio a. Nunez, Matthew Z. Farber, Laurence G. Rahme, and Madhu S. Malo of MGH’s Department of Surgery; Raza S. Hoda of the Pathology Department at MGH; Vanita Chopra of the Department of Neurology at MGH.