Rotavirus is responsible for more than 130,000 deaths annually in infants and children younger than five years. The virus causes severe, dehydrating diarrhea as it replicates in viral factories called viroplasms that form inside infected cells. Viroplasms have been difficult to study because they normally form very quickly, but a serendipitous observation led researchers at Baylor College of Medicine to uncover new insights into the formation of viroplasms.
The researchers created a mutant rotavirus that unexpectedly replicated much slower than the original virus, allowing them to observe the first steps of viral assembly. The findings, published in the Journal of Virology, open new possibilities for treating and preventing this viral disease and for understanding how similar factories of other viruses work.
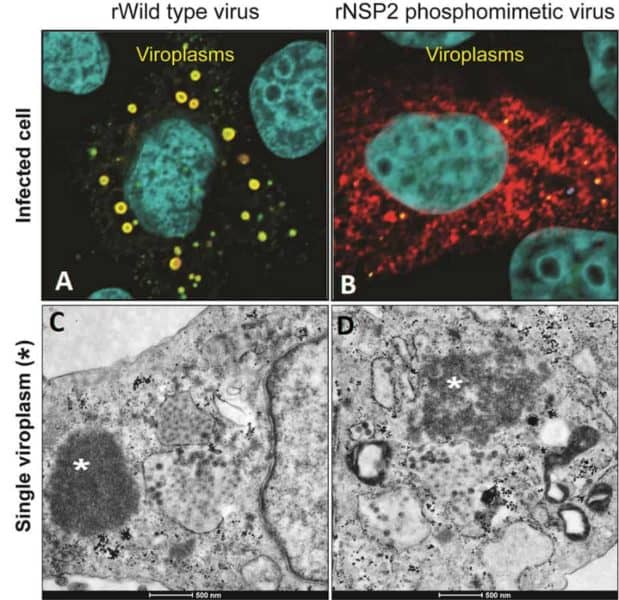
“The formation of viroplasms is indispensable for a successful rotavirus infection. They form quickly inside infected cells and are made of both viral and cellular proteins that interact with lipid droplets, but the details of how the parts are put together are still not clear,” said first author Dr. Jeanette M. Criglar, a former postdoctoral trainee and now staff scientist in the Department of Molecular Virology and Microbiology at Baylor in Dr. Mary Estes’s lab.

To get new insights into the formation of viroplasms, Criglar and her colleagues studied NSP2, one of the viral proteins that is required for the virus to replicate. Without it, neither viroplasms nor new viruses would form.
Like all proteins, NSP2 is made of amino acids strung together like beads on a necklace. ‘Bead’ 313 is the amino acid serine. Importantly, serine 313 is phosphorylated – it has a phosphate chemical group attached to it. Protein phosphorylation is a mechanism cells use to regulate protein activity. It works like an on-and-off switch, activating or deactivating a protein. Here, the researchers evaluated the role NSP2’s phosphorylation of serine 313 plays on viroplasm formation.
A serendipitous finding
Using a recently developed reverse genetics system, Criglar and her colleagues generated a rotavirus carrying an NSP2 protein with a mutation in amino acid 313, called a phosphomimetic mutation, by changing serine to aspartic acid. The name phosphomimetic indicates that the mutant protein mimics the phosphorylated protein in the original rotavirus. Reverse genetics starts with a protein and works backward to make the mutant gene, which then is made part of the virus to study the function of the protein on viral behavior.
“In laboratory experiments, our phosphomimetic mutant protein crystalized faster than the original, within hours as opposed to days,” Criglar said.
Surprisingly, when compared to non-mutant rotavirus, the phosphomimetic virus was slow to make viroplasms and to replicate.”

“This is not what we expected. We thought that rotavirus with the mutant protein also would replicate faster,” said Estes, Cullen Foundation Endowed Chair and Distinguished Service Professor of molecular virology and microbiology at Baylor. “We took advantage of the delay in viroplasm formation to observe very early events that have been difficult to study.”
Early steps: NSP2 and lipid droplets come together
The researchers discovered that one of the first steps in viroplasm formation is the association of NSP2 with lipid droplets, indicating that NSP2 phosphorylated on position 313 alone can interact with the droplets, without interacting with other components of the viroplasm.
Lipid droplets are an essential part of viroplasms. It is known that rotavirus coaxes infected cells to produce the droplets, but how it does it is unknown. The new findings suggest that rotavirus may be using phosphorylated NSP2 to trigger lipid droplet formation.
“It was very exciting to see that just changing a single amino acid in the NSP2 protein affected the replication of the whole virus,” Criglar said. “The phosphomimetic change altered the dynamics of viral replication without killing the virus. We can use this mutant rotavirus to continue investigating the sequence of events leading to viroplasm formation, including a long-standing question in cell biology about how lipid droplets form.”
“This is the first study in our lab that has used the reverse genetics system developed for rotavirus by Kanai and colleagues in Japan, and that’s very exciting for me,” Estes said. “There have been very few papers that use the system to ask a biological question, and ours is one of them.”
Other contributors to this work include Sue E. Crawford, Boyang Zhao, Hunter G. Smith and Fabio Stossi, all at Baylor.
This work was primarily supported by National Institutes of Health (NIH) grant R01 AI080656. Further support was provided by the Advanced Technology Core Laboratories at Baylor, specifically the Integrated Microscopy Core with funding from NIH (DK56338, CA125123 andES030285), CPRIT (RP150578, RP170719), the Dan L Duncan Comprehensive Cancer Center and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.