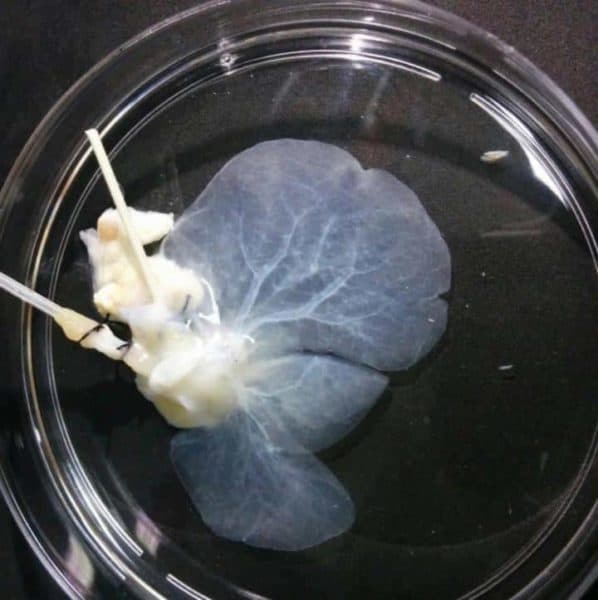
Researchers at the Human Genome and Stem Cell Research Center (HUG-CELL), hosted by the University of São Paulo’s Institute of Biosciences (IB-USP) in Brazil, have developed a technique to reconstruct and produce livers in the laboratory.
The proof-of-concept study was conducted with rat livers. In the next stage of their research, the scientists will adapt the technique for the production of human livers in order in future to increase the supply of these organs for transplantation.
The study was supported by FAPESP and is reported in an article published in Materials Science and Engineering: C. “The plan is to produce human livers in the laboratory to scale. This will avoid having to wait a long time for a compatible donor and reduce the risk of rejection of the transplanted organ,” Luiz Carlos de Caires-Júnior, first author of the article, told Agência FAPESP. He is a postdoctoral fellow of HUG-CELL, one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
The methodology is based on decellularization and recellularization, tissue bioengineering techniques developed in recent years to produce organs for transplantation. An organ from a deceased donor, in this case the liver, is treated with various solutions containing detergents or enzymes to remove all the cells from the tissue, leaving only the extracellular matrix with the organ’s original structure and shape. The extracellular matrix is then seeded with cells taken from the patient. The technique avoids immune system reactions and the risk of rejection in the long term.
“It’s comparable to transplanting a ‘reconditioned’ liver. It won’t be rejected because it uses the patient’s own cells, and there’s no need to administer immunosuppressants,” said Mayana Zatz, HUG-CELL’s principal investigator and last author of the article.
The technique can also be used to reconstitute organs considered borderline and non-transplantable, increasing the supply of organs for patients on the waiting list, Caires-Júnior explained.
“Many organs available for transplantation can’t actually be used because the donor has died in a traffic accident. The technique can be used to repair them, depending on their status,” he said.
The decellularization process, however, removes the main components of the extracellular matrix, such as molecules that tell the cells to multiply and form blood vessels, for example. This weakens cell adhesion to the extracellular matrix and compromises recellularization.
To surmount this obstacle, the HUG-CELL researchers enhanced the technique by introducing an extra stage between decellularization and recellularization.
After isolating and decellularizing rat livers, they injected into the extracellular matrix a solution rich in molecules such as SPARC and TGFB1, proteins produced by liver cells grown in a laboratory in a conditioned medium. These proteins are essential to a healthy liver as they tell liver cells to proliferate and form blood vessels.
“Enrichment of the extracellular matrix with these molecules lets it become much more similar to that of a healthy liver,” Caires-Júnior said.
Rat liver extracellular matrices were treated with the solution, and hepatocytes, endothelial cells, and mesenchymal cells were introduced into the material. The mesenchymal cells were derived from human induced pluripotent stem cells (iPSCs), produced by reprogramming adult skin cells (or cells from other easily accessible tissues) into an embryonic-like pluripotent state.
“The study shows it’s possible to induce human stem cell differentiation in cell lineages that are part of a liver and use these cells to reconstruct the organ so that it’s functional. It’s proof of concept, the first demonstration that the technique works,” Zatz said.
The liver cells were injected with a syringe pump into rat liver extracellular matrices to produce an organ with human characteristics. It grew for five weeks in an incubator that simulated the conditions in the human body. Analysis showed that extracellular matrix enrichment with SPARC and TGFB1 significantly improved recellularization.
“The treatment made the liver cells grow and function more vigorously,” Caires-Júnior said. “We plan to build a bioreactor to decellularize human livers and study the possibility of producing them to scale in the laboratory.”
The technique can be adapted to produce other organs, such as lungs, hearts, and skin, he added.
Organ factories
The project is part of one of the research lines pursued by HUG-CELL to produce or reconstruct transplant organs using different techniques.
Through a project conducted in partnership with pharmaceutical company EMS and supported by FAPESP (São Paulo Research Foundation) under the auspices of its Research Partnership for Technological Innovation Program (PITE), the HUG-CELL researchers aim to modify pig organs such as kidneys, hearts and skin for transplantation into humans (read more at: agencia.fapesp.br/29771/).
Pig livers would be rejected if they were transplanted into humans, so the researchers are pursuing other strategies, such as 3D printing (read more at: agencia.fapesp.br/32217/), as well as decellularization and recellularization.
“These are complementary approaches. We expect to see transplant organ factories in future,” Zatz said.
About São Paulo Research Foundation (FAPESP)
The São Paulo Research Foundation (FAPESP) is a public institution with the mission of supporting scientific research in all fields of knowledge by awarding scholarships, fellowships and grants to investigators linked with higher education and research institutions in the State of São Paulo, Brazil. FAPESP is aware that the very best research can only be done by working with the best researchers internationally. Therefore, it has established partnerships with funding agencies, higher education, private companies, and research organizations in other countries known for the quality of their research and has been encouraging scientists funded by its grants to further develop their international collaboration. You can learn more about FAPESP at http://www.