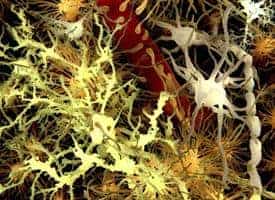
The brain’s neurons tend to get most of the scientific attention, but a set of cells around them called astrocytes – literally, star-shaped cells – are increasingly being viewed as crucial players in guiding a brain to become properly organized.
Specifically, astrocytes, which form about half the mass of a human brain, seem to guide the formation of synapses, the connections between neurons that are formed and remodeled as we learn and remember.
A new study from Duke and UNC scientists has discovered a crucial protein involved in the communication and coordination between astrocytes as they build synapses. Lacking this molecule, called hepaCAM, astrocytes aren’t as sticky as they should be, and tend to stick to themselves rather than forming connections with their fellow astrocytes.
This finding, in studies on mice with the gene for hepaCAM knocked out of their astrocytes, is an important clue in efforts to understand several brain disorders, including cognitive decline, epilepsy and autism spectrum disorders. The work appears June 24 in the journal Neuron.
A rare disorder called megalencephalic leukoencephalopathy (MLC) is also known to be caused by a mutation in the hepaCAM gene, and this work might provide answers about what exactly has gone wrong. MLC is a developmental disorder that grows progressively worse, causing macrocephaly (a large head), swelling of the brain’s white matter, intellectual disability and epilepsy.
By removing hepaCAM selectively from astrocytes to see what it does, “we sort of made the cells into introverts,” said senior author Cagla Eroglu, an associate professor of cell biology at the Duke University School of Medicine. “They’re normally wanting to reach out, but without hepaCAM, they started to hug themselves instead.”
“If the astrocyte makes junctions to its neighbors, then you start to have a network,” Eroglu said. “To make a functional brain, you need a functional astrocytic network.”
The researchers zeroed in on hepaCAM by looking for genes that are highly active in astrocytes and which have been implicated in brain dysfunction. They partnered with another group working on hepaCAM at the University of Barcelona, but that group has been looking at the molecule for its role in regulating chloride signaling channels in astrocytes.
The Duke group found that removing hepaCAM from astrocytes led to a synaptic network that was too easily excited and not as well dampened. “The effect on the inhibitory synapses was the strongest,” said first author Katie Baldwin, who recently became an assistant professor of cell biology and physiology at the University of North Carolina at Chapel Hill. “You’re putting the inhibition down and the excitation up, so that really could point to a mechanism for epilepsy.”
Baldwin, who did this work as a postdoctoral researcher in Eroglu’s lab, is planning to further pursue these questions in her new lab at UNC, testing whether hepaCAM-deficient mice behave differently or have changes in learning and memory, or whether they exhibit the stress and social anxiety that are markers of autism spectrum disorders. She said they might also reintroduce the disease-mutation versions of the protein to mice that were born without it to see what effects it has.
“We know hepaCAM is interacting with itself between two astrocytes, but we don’t know what it’s interacting with at the synapse,” Baldwin said. “We don’t know if it could be interacting with hepaCAM which is also found in the neurons, or if it could be some other protein that we don’t know about yet.”
This research was supported by the U.S. National Institutes of Health (R01DA047258, R01NS102237, F32 NS100392), Foerster-Bernstein Family, The Hartwell Foundation, and the European Research Council (725780 LinPro).
CITATION: “HepaCAM Controls Astrocyte Self-Organization and Coupling,” Katherine Baldwin, Christabel Tan, Samuel Strader, Changyu Jiang, Justin Savage, Xabier Elorza-Vidal, Ximena Contreras, Thomas Ruelicke, Simon Hippenmayer, Raul Estevez, Ru-Rong Ji and Cagla Eroglu. Neuron, June 24, 2021. DOI: 10.1016/j.neuron.2021.05.025