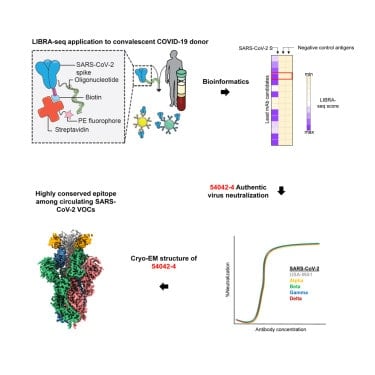
A technology developed at Vanderbilt University Medical Center has led to the discovery of an “ultra-potent” monoclonal antibody against multiple variants of SARS-CoV-2, the virus responsible for COVID-19, including the delta variant.
The antibody has rare characteristics that make it a valuable addition to the limited set of broadly reactive antibody therapeutic candidates, researchers reported in the journal Cell Reports.
The technology, called LIBRA-seq, has helped speed up the discovery of antibodies that can neutralize SARS-CoV-2. It also enables researchers to screen antibodies against other viruses that have not yet caused human disease but which have a high potential of doing so.
“This is one way to proactively build a repertoire of potential therapeutics” against future outbreaks, said Ivelin Georgiev, PhD, director of the Vanderbilt Program in Computational Microbiology and Immunology and associate director of the Vanderbilt Institute for Infection, Immunology and Inflammation.
“The pathogens keep evolving, and we’re basically playing catch-up,” said Georgiev, associate professor of Pathology, Microbiology & Immunology and Computer Science, and a member of the Vanderbilt Vaccine Center.
A more proactive approach that anticipates future outbreaks before they occur is needed to prevent a repeat of COVID-19, “or something worse happening in the future,” he said.
In their report, Georgiev and his colleagues describe the isolation of a monoclonal antibody from a patient who had recovered from COVID-19 that “shows potent neutralization” against SARS-CoV-2. It also is effective against variants of the virus that are slowing efforts to control the pandemic.
The antibody has uncommon genetic and structural characteristics that distinguish it from other monoclonal antibodies commonly used to treat COVID-19. The thought is that SARS-CoV-2 will be less likely to mutate to escape an antibody it hasn’t “seen” before.
LIBRA-seq stands for Linking B-cell Receptor to Antigen Specificity through sequencing. It was developed in 2019 by Ian Setliff, PhD, a former graduate student in Georgiev’s lab who now works in the biotechnology industry, and by Andrea Shiakolas, a current Vanderbilt graduate student.
Setliff wondered if he could map the genetic sequences of antibodies and the identities of specific viral antigens, the proteins markers that antibodies recognize and attack, simultaneously and in a high-throughput way. The goal was to find a faster way of identifying antibodies that will hone in on a specific viral antigen.
With the help of VUMC’s core genomics laboratory, Vanderbilt Technologies for Advanced Genomics (VANTAGE), the Vanderbilt Flow Cytometry Shared Resource, and Vanderbilt University’s Advanced Computing Center for Research and Education (ACCRE), Georgiev put Setliff’s idea to the test. It worked.
The efforts led by Setliff and Shiakolas culminated in a manuscript describing proof-of-concept development of the LIBRA-seq technology that was published in the journal Cell in 2019.
“It would have been impossible three or four years ago to move at the speed that we are right now,” Georgiev said. “A lot has changed in a very short period of time when it comes to monoclonal antibody discovery as well as vaccine development.”
There is no time to lose. “If we give the virus enough time,” he said, “there will so many other variants that arise,” one or more of which–by evading current vaccines–may be even worse than the delta variant.
“That’s exactly why you need to have as many options as possible,” Georgiev said. The antibody described in this paper “basically gives you another tool in the toolbox.”