Electrocatalytic carbon dioxide reduction (CO2RR) is an effective means of CO2 resource utilization. The current developed catalysts can effectively catalyze CO2RR to prepare a variety of carbon-based fuels such as formic (HCOOH) which is most likely to be commercialized in the future.
However, the current catalyst present particle agglomerate, active-phase change and element dissolution during the high-speed electrolysis, resulting in the rapid drop of HCOOH selectivity. It is necessary to develop catalysts with both activity and stability to realize the industrial preparation of electrocatalytic CO2 to HCOOH.
A research team led by Prof. GAO Minrui from University of Science and Technology of China (USTC) of the Chinese Academy of Sciences stabilized the catalyst structure by strengthening the covalent property of materials. With the obtained indium sulfide catalyst, the selectivity of formic acid product can remain about 97%. This work was published in Nature Communications.
The researchers improved the covalence of indium sulfur bond and effectively inhibited the loss of active component, sulfur, in the catalyst by introducing zinc (Zn) into indium sulfide. Utilizing the same synthesis strategy, they prepared In2S3 and ZnIn2S4 catalysts to explore its efficiency.
Electrochemical performance test showed that the structure of ZnIn2S4, modulated by Zn, presented great HCOO– Faraday efficiency and partial current density in neutral electrolytic environment. In particular, the incorporation of Zn enabled ZnIn2S4 to have excellent catalytic stability. In the uninterrupted continuous electrolysis process, the catalyst could stably catalyze the formation of HCOO– at industrial current density over 60 hours, and maintain the Faraday efficiency at about 97%.
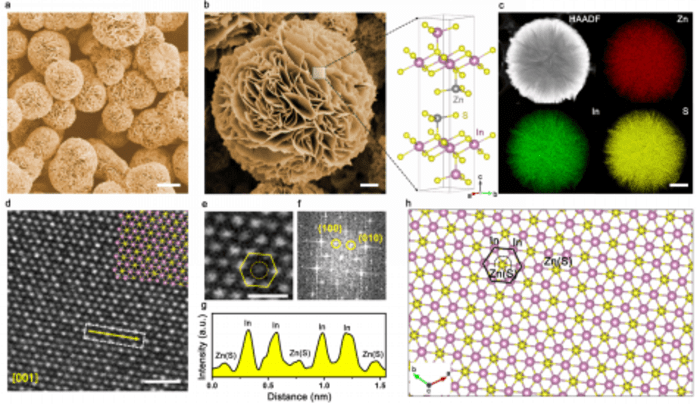
Furthermore, the researchers conducted rigorous structural stability tests on the catalyst. Through X-ray diffraction and scanning electron microscopy, they found that the phase and morphology of ZnIn2S4 catalyst were well maintained even when the current density was relatively higher. Whereas, In2S3 was completely transformed into a metallic In, and S was completely lost at relatively low current density. In this condition, the loss of S led to the complete structure collapse.
They also used X-ray photoelectron spectroscopy and STEM-EDX elemental analysis to confirm the serious loss of S of In2S3 during electrolysis. On the contrary, the content and chemical state of each element (Zn, ln and Si) in ZnIn2S4 structure hardly changed. With the help of computer simulation, they found that in ZnIn2S4, the bond fracture between in ln(Zn) and S was unfavorable in dynamics and the dissolution of S element was blocked. This simulation result explained the internal mechanism of high current electrolysis stability.
This work improved the internal stability of the catalyst by introducing foreign elements to enhance the covalency of the material, which provides a new idea for the design and creation of CO2RR catalyst for long-time industrial preparation.