With help from the best tweezers in the world a team of researchers from the University of Copenhagen has shed new light on a fundamental mechanism in all living cells that helps them explore their surroundings and even invade tissue. Their discovery could have implications for research into cancer, neurological disorders and much else.
Using octopus-like tentacles, a cell pushes toward its target, a bacterium, like a predator tracking down its prey. The scene could be playing out in a nature programme. Instead the pursuit is being observed at the nano-scale through a microscope at the University of Copenhagen’s Niels Bohr Institute. The microscope recording shows a human immune cell pursuing and then devouring a bacterium.
With their new study, a team of Danish researchers has added to the world’s understanding of how cells use octopus-like tentacles called filopodia to move around in our bodies. This discovery about how cells move had never been addressed. The study is being published today in the renowned journal, Nature Communications.
“While the cell doesn’t have eyes or a sense of smell, its surface is equipped with ultra-slim filopodia that resemble entangled octopus tentacles. These filopodia help a cell move towards a bacterium, and at the same time, act as sensory feelers that identify the bacterium as a prey,” explains Associate Professor Poul Martin Bendix, head of the laboratory for experimental biophysics at the Niels Bohr Institute.
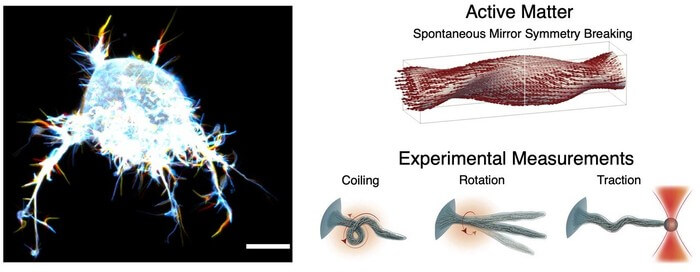
The discovery is not that filopodia act as sensory devices – which was already well established – but rather about how they can rotate and behave mechanically, which helps a cell move, as when a cancer cell invades new tissue.
“Obviously, our results are of interest to cancer researchers. Cancer cells are noted for their being highly invasive. And, it is reasonable to believe that they are especially dependent on the efficacy of their filopodia, in terms of examining their surroundings and facilitating their spread. So, it’s conceivable that by finding ways of inhibiting the filopodia of cancer cells, cancer growth can be stalled,” explains Associate Professor Poul Martin Bendix.
For this reason, researchers from the Danish Cancer Society Research Center are a part of the team behind the discovery. Among other things, the cancer researchers are interested in whether switching off the production of certain proteins can inhibit the transport mechanisms which are important for the filopodia of cancer cells.
The cell’s engine and cutting torch
According to Poul Martin Bendix, the mechanical function of filopodia can be compared to a rubber band. Untwisted, a rubber band has no power. But if you twist it, it contracts. This combination of twisting and contraction helps a cell move directionally and makes the filopodia very flexible.
“They’re able to bend — twist, if you will — in a way that allows them to explore the entire space around the cell, and they can even penetrate tissues in their environment,” says lead author, Natascha Leijnse.
The mechanism discovered by the Danish researchers appears to be found in all living cells. Besides cancer cells, it is also relevant to study the importance of filopodia in other types of cells, such as embryonic stem cells and brain cells, which are highly dependent on filopodia for their development.
Studying cells with the best tweezers in the world
The project involved interdisciplinary collaboration at the Niels Bohr Institute, where Associate Professor Amin Doostmohammadi, who heads a research group that simulates biologically active materials, contributed with the modelling of filopodia behaviour.
“It is very interesting that Amin Doostmohammadi could simulate the mechanical movements we witnessed through the microscope, completely independent of chemical and biological details,” explains Poul Martin Bendix.
The main reason that the team succeeded in being the first to describe the mechanical behaviour of filopodia is that NBI has unique equipment for this type of experiment, as well as skilled researchers with tremendous experience working with optical tweezers. When an object is extraordinarily small, holding onto it mechanically becomes impossible. However, it can be held and moved using a laser beam with a wavelength carefully calibrated to the object being studied. This is called an optical tweezers.
“At NBI, we have some of the world’s best optical tweezers for biomechanical studies. The experiments require the use of several optical tweezers and the simultaneous deployment of ultra-fine microscopy,” explains Poul Martin Bendix.
Leading the study alongside Poul Martin Bendix and Assistant Professor Natascha Leijnse was NBI Technical Scientist Younes Barooji. The article on cell filopodia is published today in Nature Communications.