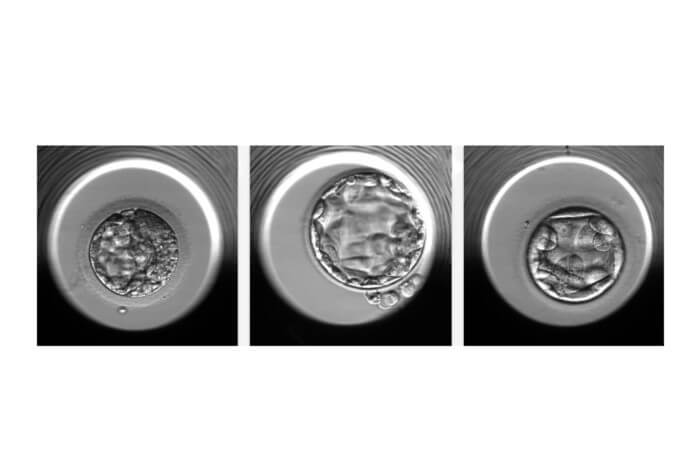
An artificial intelligence (AI) algorithm can determine non-invasively, with about 70% accuracy, whether an in vitro fertilized (IVF) embryo has a normal or abnormal number of chromosomes, according to a new study from researchers at Weill Cornell Medicine.
This condition, known as aneuploidy, is a major reason that embryos derived from IVF fail to implant or result in a healthy pregnancy. Currently, one of the methods for detecting aneuploidy involves the biopsy-like sampling and genetic testing of cells from an embryo, which is both invasive and adds cost to the IVF process. The new AI algorithm, called STORK-A, can help predict aneuploidy without these disadvantages. It operates by analyzing microscope images of the embryo and incorporates information about maternal age and the IVF clinic’s scoring of the embryo’s appearance.
“Our hope is that we’ll ultimately be able to predict aneuploidy in a completely non-invasive way, using artificial intelligence and computer vision techniques,” said Dr. Iman Hajirasouliha, senior author of the study and associate professor of computational genomics and of physiology and biophysics at Weill Cornell Medicine.
Currently, fertility clinic staff use microscopy to assess embryos for large-scale abnormalities that may indicate poor viability. They may also use a biopsy method called preimplantation genetic testing for aneuploidy (PGT-A), predominantly in women over the age of 37, to obtain information about the chromosomes. To develop a computational approach to embryo assessment, investigators from the Center for Reproductive Medicine teamed up with colleagues in the Englander Institute. In a previous study, they developed an AI algorithm called STORK that was able to assess embryo quality as well as IVF clinic staff.
For the current study, they developed STORK-A as a potential replacement for PGT-A or as a more selective way of deciding which embryos should undergo PGT-A testing. STORK-A uses microscope images of embryos taken at five days past fertilization, as well as clinic staff’s scoring of embryo quality, maternal age, and other information normally gathered during the IVF process. The AI algorithm “learns” to correlate certain features of the data, often too subtle for the human eye, with the likelihood of aneuploidy. The team trained STORK-A on a dataset of 10,378 blastocysts for which ploidy status was already known.
From its performance, the algorithm’s accuracy in predicting aneuploid versus normal-chromosome “euploid” embryos was almost 70% (69.3%). In predicting aneuploidy involving more than one chromosome, known as complex aneuploidy, versus euploidy, STORK-A was 77.6% accurate. The algorithm was then tested on independent datasets, including one from an IVF clinic in Spain, and similar results were obtained.
The researchers believe that STORK-A has the potential to improve the success rate of IVF and achieve more successful pregnancies with fewer embryo transfers. They plan to further develop and test the algorithm to increase its accuracy and determine its potential for clinical use.