Earth’s potassium arrived by meteoritic delivery service finds new research led by Carnegie’s Nicole Nie and Da Wang. Their work, published in Science, shows that some primitive meteorites contain a different mix of potassium isotopes than those found in other, more-chemically processed meteorites. These results can help elucidate the processes that shaped our Solar System and determined the composition of its planets.
“The extreme conditions found in stellar interiors enable stars to manufacture elements using nuclear fusion,” explained Nie, a former Carnegie postdoc now at Caltech. “Each stellar generation seeds the raw material from which subsequent generations are born and we can trace the history of this material across time.”
Some of the material produced in the interiors of stars can be ejected out into space, where it accumulates as a cloud of gas and dust. More than 4.5 billion years ago, one such cloud collapsed in on itself to form our Sun.
The remnants of this process formed a rotating disk around the newborn star. Eventually, the planets and other Solar System objects coalesced from these leftovers, including the parent bodies that later broke apart to become asteroids and meteorites.
“By studying variations in the isotopic record preserved within meteorites, we can trace the source materials from which they formed and build a geochemical timeline of our Solar System’s evolution,” added Wang, who is now at Chengdu University of Technology.
Each element contains a unique number of protons, but its isotopes have varying numbers of neutrons. The distribution of different isotopes of the same element throughout the Solar System is a reflection of the makeup of the cloud of material from which the Sun was born. Many stars contributed to this so-called solar molecular cloud, but their contributions were not uniform, which can be determined by studying the isotopic content of meteorites.
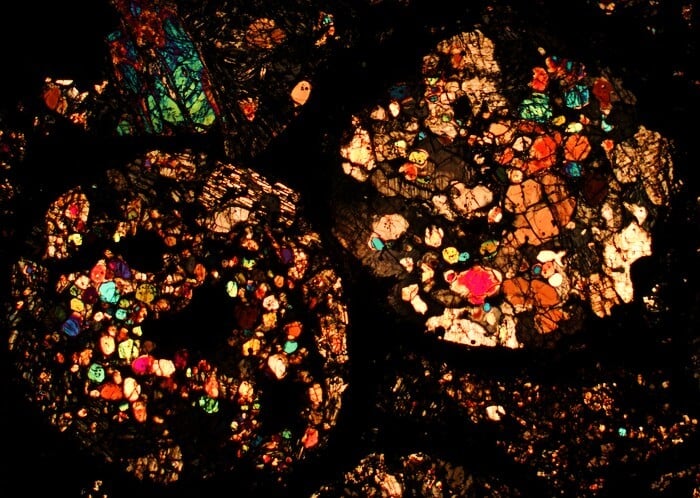
Wang and Nie—along with Carnegie colleagues Anat Shahar, Zachary Torrano, Richard Carlson, and Conel Alexander—measured the ratios of three potassium isotopes in samples from 32 different meteorites.
Potassium is particularly interesting because it’s what’s called a moderately volatile element, which are named for having relatively low boiling points that cause them to evaporate fairly easily. As a result, it’s challenging to look for patterns that predate the Sun in the isotopic ratios of volatiles—they just don’t stick around in the hot star-forming conditions long enough to maintain an easily readable record.
“However, using very sensitive and suitable instruments, we found patterns in the distribution of our potassium isotopes that were inherited from pre-solar materials and differed between types of meteorites,” Nie said.
They found that some of the most primitive meteorites called carbonaceous chondrites, which formed in the outer Solar System, contained more potassium isotopes that were produced by huge stellar explosions, called supernovae. Whereas other meteorites—those that most frequently crash to Earth, called non-carbonaceous chondrites—contain the same potassium isotope ratios seen on our home planet and elsewhere in the inner Solar System.
“This tells us that, like a poorly mixed cake batter, there wasn’t an even distribution of materials between the outer reaches of the Solar System where the carbonaceous chondrites formed, and the inner Solar System, where we live,” concluded Shahar.
For years, Carnegie Earth and planetary scientists have worked to reveal the origins of Earth’s volatile elements. Some of these elements may have been transported here all the way from the outer Solar System on the backs of carbonaceous chondrites. However, since the pattern of pre-solar potassium isotopes found in non-carbonaceous chondrites matched that seen on Earth, these meteorites are the probable source of our planet’s potassium.
“It is only recently that scientists challenged a once long-held belief that the conditions in the solar nebula that birthed our Sun were hot enough to burn off all volatile elements,” Shahar added. “This research provides fresh evidence that volatiles could survive the Sun’s formation.”
More research is needed to apply this new knowledge to our models of planet formation and see if it adjusts any long-held beliefs about how Earth and its neighbors came into being.