The development of biomaterials for artificial organs and tissues is active due to an increase in accidental injuries and chronic diseases, along with the entry into a super-aged society. 3D bioprinting technology, which uses cells and biomaterials to create three-dimensional artificial tissue structures, has recently gained popularity. However, commonly used hydrogel-based bioinks can cause cytotoxicity due to the chemical crosslinking agent and ultraviolet light that connect the molecular structure of photocuring 3D-printed bioink.
Dr. Song Soo-chang’s research team at the Center for Biomaterials, Korea Institute of Science and Technology (KIST, President Yoon Seok-jin), revealed the first development of poly(organophosphazene) hydrogel-based temperature-sensitive bioink that stably maintained its physical structure only by temperature control without photocuring, induced tissue regeneration, and then biodegraded in the body after a certain period of time.
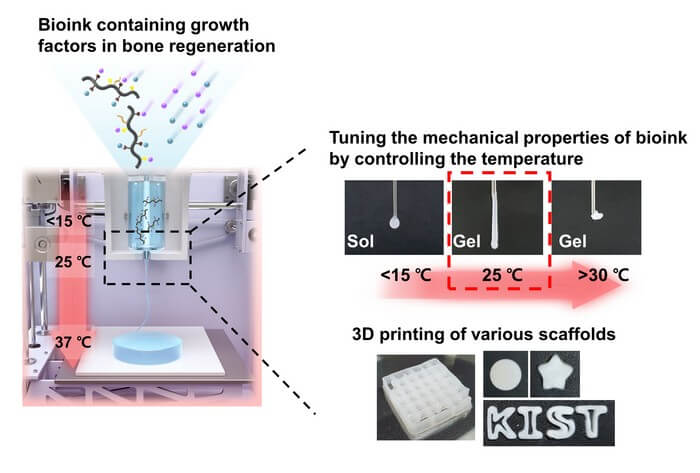
Current hydrogel-based bioinks must go through a photocuring process to enhance the mechanical properties of the 3D scaffold after printing, with a high risk of adverse effects in the human body. In addition, there have been possibility of side effects by transplanting externally cultured cells within bioink to increase the tissue regeneration effect. Accordingly, the research team developed a new bioink material using a temperature-sensitive poly(organophosphazene) hydrogel, which existed in a liquid form at low temperatures and changed to a hard gel at body temperature. This enabled the regeneration of tissues only by temperature control without chemical crosslinking agents or UV irradiation and the manufacture of a three-dimensional scaffold with a physically stable structure, minimizing the possibility of immune adverse effects in the human body.
The developed bioink also had a molecular structure that could interact with growth factors, which were proteins that help in tissue regeneration to preserve growth factors that regulated cell growth, differentiation, and immune responses for a long period of time. The research team was able to maximize the effect of tissue regeneration by creating an environment in which cell differentiation could be autonomously regulated within the 3D scaffold printed with bioink.
The research team fabricated the 3D scaffold by printing it with a 3D bioprinter using bioink containing transforming growth factor beta 1 (TGF-β1) and bone morphogenetic protein-2 (BMP-2), which were required for cell infiltration and bone regeneration, and conducted an experiment by implanting it into a damaged bone in a rat. As a result, cells from the surrounding tissue were migrated into the scaffold, and the defected bone was regenerated to a normal tissue level, and the implanted 3D scaffold slowly biodegraded in the body over 42 days.
Dr. Song Soo-Chang of KIST said, “The research team has transferred technology for the thermo-sensitive polyphosphazene hydrogel to NexGel Biotech Co., Ltd. in June 2022, and the development of products such as bone graft materials and cosmetic fillers is underway.” “As the bioink developed this time has different physical properties, follow-up research to apply it to the regeneration of other tissues besides bone tissue is being conducted, and we expect to finally be able to commercialize bioink tailored to each tissue and organ,” he said.