Researchers at the Tokyo Institute of Technology have unveiled a novel boron agent that significantly enhances the efficiency of boron neutron capture therapy (BNCT) for glioblastoma, a highly aggressive form of brain tumor.
The agent is unique in that it can be selectively absorbed by brain tumor cells, possesses extended blood retention, and requires low dosages for administration. Encouraging results have emerged from experiments conducted on cell cultures, mice, and rats, underlining the potential of this innovative agent in radiotherapy.
GBM is a rapidly proliferating brain tumor that originates from glial cells, the supportive tissue of the brain. The swift growth rate of the tumor often renders surgical removal ineffective, thereby emphasizing the importance of radiotherapy. BNCT has been in the limelight as a viable alternative treatment for GBM.
The concept of BNCT leverages the high affinity of boron-10 (10B) atoms for low-energy neutrons. Absorption of a neutron by 10B initiates nuclear reactions that release high-energy particles, which subsequently damage the adjacent biological tissue. A crucial element of BNCT is to concentrate a significantly higher number of 10B atoms in tumor cells than in healthy ones. However, this has been a considerable challenge, restricting the wide-scale use of BNCT and contributing to lower survival rates.
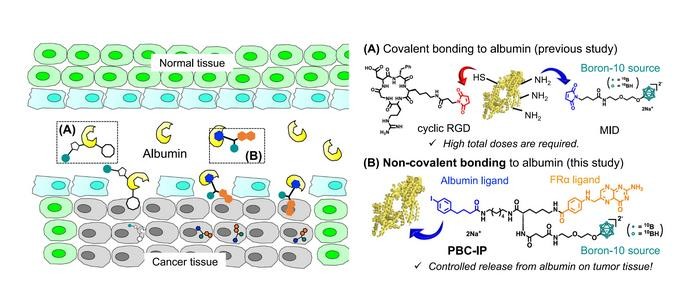
In response to these challenges, a research team, inclusive of Professor Hiroyuki Nakamura from Tokyo Tech, has engineered a promising boron agent for BNCT. The agent, Pteroyl-closo-dodecaborate conjugated with a 4-(p-iodophenyl)butyric acid moiety (PBC-IP), underwent rigorous testing in their study recently published in the Journal of Controlled Release.
PBC-IP comprises three principal functional groups: a boron group carrying twelve 10B atoms, a ligand designed to bind to the folate receptor α (FRα), and a 4-(p-iodophenyl)butyric acid moiety. FRα, largely absent in normal cells, is considerably overexpressed in various cancers, including GBM, and serves as the entry point for PBC-IP into tumor cells. The 4-(p-iodophenyl)butyric acid moiety binds PBC-IP to albumin, a widespread carrier protein in blood that transports substances throughout the body. By binding to naturally occurring albumin, PBC-IP can interact directly with tumor cells, boosting its cellular uptake and extending the boron agent’s blood retention, thus possibly reducing the required dose.
Various experiments were conducted to assess the effectiveness of PBC-IP for BNCT. The findings revealed that PBC-IP accumulated in GBM cell cultures 10–20 times more than L-4-boronophenylalanine (BPA), a boron agent approved clinically in Japan. Importantly, PBC-IP showed no signs of toxicity to cells on its own, evidencing its safety. “Likewise, PBC-IP administered intravenously to the human GBM xenograft model demonstrated a higher accumulation of boron in tumors than BPA, effectively inhibiting tumor growth following thermal neutron irradiation,” emphasized Prof. Nakamura.
These encouraging results were also replicated in vivo in GBM xenograft and rat glioma models. Three hours after the completion of convection-enhanced delivery (CED), the PBC-IP administered in the rat model achieved tumor-to-normal brain and tumor-to-blood boron ratios of 37.8 and 94.6, respectively. Prof. Nakamura conveyed the promising outcomes: “Survival rates at 180 days were 50% and 70% following BNCT with PBC-IP only and PBC-IP in combination with BPA, respectively. There were no residual brain tumors.”
In sum, the innovative boron agent may signify a game-changer in radiotherapy for GBM, with preclinical studies underway. Here’s to hoping these strides in research can pave the way for improved treatments against GBM and other cancers.