With an estimated 5.8 million Americans currently living with Alzheimer’s disease, the need for a cure has never been more pressing. The lack of a comprehensive understanding of the disease’s causes has hindered progress in finding effective treatments. However, a recent study from Scripps Research offers new insights into the molecular drivers that may contribute to the progression of this debilitating neurodegenerative disorder.
Exploring the Electrical Activity and Protein Levels of Single Neurons
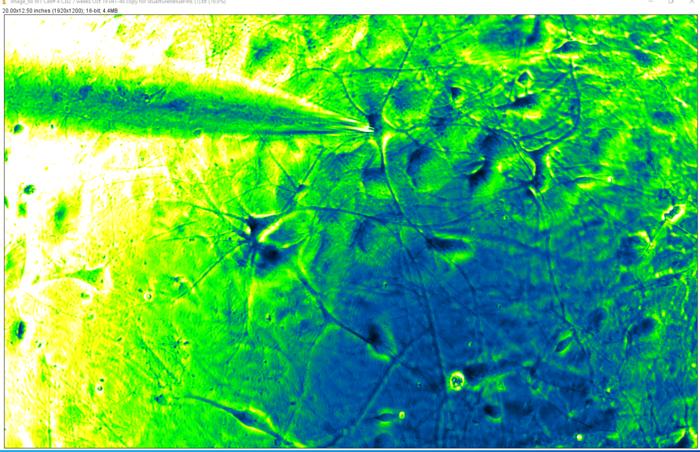
The groundbreaking study, published in Advanced Science on May 21, 2024, showcases a novel technique for studying single, living brain cells affected by Alzheimer’s disease. By measuring the electrical activity and protein levels within individual neurons, the researchers identified new molecules linked to the disease. This discovery raises hopes for the development of targeted drugs that could treat or slow the progression of Alzheimer’s in the future.
The study’s success can be attributed to the close collaboration among Scripps Research’s professors, including clinical neurologist Stuart Lipton, MD, PhD, protein expert John Yates, III, PhD, and bioinformaticist Nicholas Schork, PhD. Their combined expertise enabled the development of this biotechnology feat, which Lipton describes as “mind-boggling.”
Connecting Hyperexcitability to Abnormal Protein Levels
Previous research has shown that certain neurons become overactive, or hyperexcitable, in the brains of people with Alzheimer’s. This hyperexcitability is believed to contribute to the cognitive decline associated with the disease. The new study aimed to uncover the molecular basis of this phenomenon by comparing the electrical activity and protein levels of Alzheimer’s-affected cells with those of healthy cells.
Using a system called single cell (sc)Patch-Clamp/Proteomics, the researchers analyzed approximately 150 neurons and identified nearly 50 proteins that were present at higher or lower levels in hyperexcitable Alzheimer’s cells compared to healthy cells. These proteins were involved in various neuronal functions, including redox modulation, energy metabolism, and inflammation.
The study’s findings not only confirm the involvement of some previously known Alzheimer’s-associated proteins but also reveal many new molecules that could serve as potential targets for drug development. Lipton and his team plan to conduct follow-up studies on some of these molecules and expand the use of scPatch-Clamp/Proteomics for drug screens.
The researchers also aim to validate their findings using cerebral organoids, or “mini-brains,” derived from patients with Alzheimer’s. This approach will allow them to investigate how the dysfunction in Alzheimer’s is related to the interaction between cells, potentially leading to additional discoveries.
The implications of this study extend beyond Alzheimer’s disease, as the single-cell technique could be applied to drug discovery efforts for other neurological disorders. As Lipton notes, “This new approach to personalized medicine—based upon protein expression and electrical activity of a single Alzheimer’s neuron—could revolutionize drug discovery not only for this disease but other neurological diseases, which has lagged far behind other therapeutic areas.”