As we age, our bodies undergo a gradual decline in physiological functions, increasing the risk of developing cardiovascular, neurodegenerative, and metabolic diseases. This process is closely linked to cellular senescence, a phenomenon in which cells stop dividing but do not die, accumulating in tissues and contributing to age-related ailments.
While the elimination of senescent cells, known as senolysis, has emerged as a potential therapeutic strategy, conventional senolytic agents that directly inhibit cellular aging signals can have concerning side effects. In a recent study published in Nature Aging, researchers from Juntendo University, Japan, have discovered a promising alternative: sodium glucose co-transporter 2 (SGLT2) inhibitors.
- Canagliflozin: Brand name Invokana
- Dapagliflozin: Brand name Forxiga
- Empagliflozin: Brand name Jardiance
- Ertugliflozin: Brand name Steglatro
- Bexaglifloxin: Brand name Brenzavvy
Caloric Restriction and SGLT2 Inhibition: A Promising Link
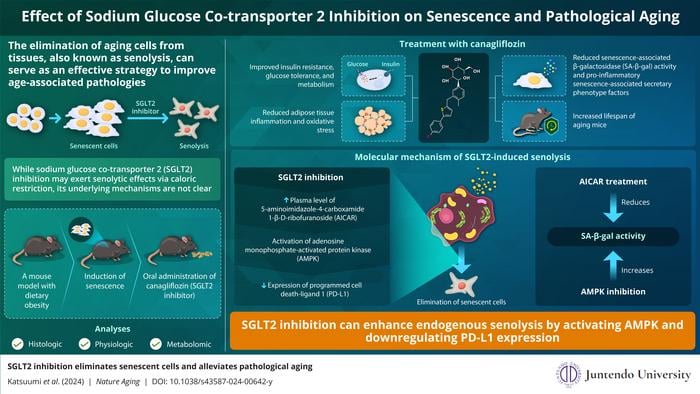
Previous research has shown that caloric restriction, or reducing average daily caloric intake, is associated with longevity and decreased accumulation of senescent cells in tissues. Additionally, inhibiting SGLT2, a key protein involved in glucose transport, effectively reduces blood glucose levels, leading to calorie loss. Professor Tohru Minamino and Dr. Goro Katsuumi, along with their research team, set out to investigate whether these effects could be extended to senescent cell removal.
“Unlike the conventional senescent cell-eliminating agents, SGLT2 inhibitors are a new class of therapeutic agents with fewer side effects as they promote senescent cell elimination by activating the immune system,” explains Prof. Minamino.
Uncovering the Molecular Mechanisms of SGLT2 Inhibition
Using a mouse model of dietary obesity, the researchers treated the mice with canagliflozin, an SGLT2 inhibitor, and examined changes in glucose metabolism and high-fat diet-induced senescence. Canagliflozin treatment significantly improved glucose metabolism, reduced insulin resistance, and decreased senescence-related markers, inflammation, and oxidative stress in adipose tissues.
Through metabolomic analysis, the researchers discovered that SGLT2 inhibition led to a significant increase in the plasma level of 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), a metabolite known to activate adenosine monophosphate-activated protein kinase (AMPK). Mice treated with AICAR exhibited reduced senescence-associated β-galactosidase (SA-β-gal) activity, similar to SGLT2 inhibition, while AMPK inhibition increased SA-β-gal activity, confirming the role of AICAR and AMPK in SGLT2 inhibition-induced senolysis.
The immune system and the immune checkpoint factor programmed cell death-1 (PD-1)/PD-L1 are also actively involved in cell senescence, with AMPK known to negatively regulate PD-L1 expression. SGLT2 inhibition with canagliflozin significantly reduced the elevated number of PD-L1-positive senescent cells in high-fat diet-fed mice, while suppression of immune cells following canagliflozin treatment increased senescent cells, indicating that the senolytic effects of canagliflozin were partly mediated by the immune system.
The study also demonstrated that SGLT2 inhibition could ameliorate specific aging-induced pathologies by removing senescent cells, improving physical activity, and increasing the lifespan of treated animals. These findings highlight the clinical potential of SGLT2 inhibition as an effective senolytic strategy in managing aging-related disorders.
“In this study, we were able to demonstrate improvement against diabetes, arteriosclerosis, premature aging, and frailty, as well as a prolonged life span in response to SGLT2 inhibition. SGLT2 inhibitors may be applied in the treatment of various aging-related diseases, including Alzheimer’s disease, in the future,” concludes Prof. Minamino.