Biomedical engineers at Duke University have created a game-changing silk-based, ultrathin membrane that can be used in organ-on-a-chip (OOC) models to better mimic the natural environment of cells and tissues within the body. When applied to a kidney organ-on-a-chip platform, the membrane enabled tissues to grow and recreate the functionality of both healthy and diseased kidneys, offering a powerful tool for disease modeling and therapeutic testing.
The research, published on June 4 in the journal Science Advances, addresses a critical issue in the design of OOC systems – the materials used to create the membranes that form the support structure for the specialized cells to grow on. Conventional polymer membranes are typically 30 to 50 microns thick, hindering communication between cells and limiting cell growth, whereas extracellular membranes in human organs are often less than one micron thick.
Silk Fibroin: A Game-Changer in Membrane Design
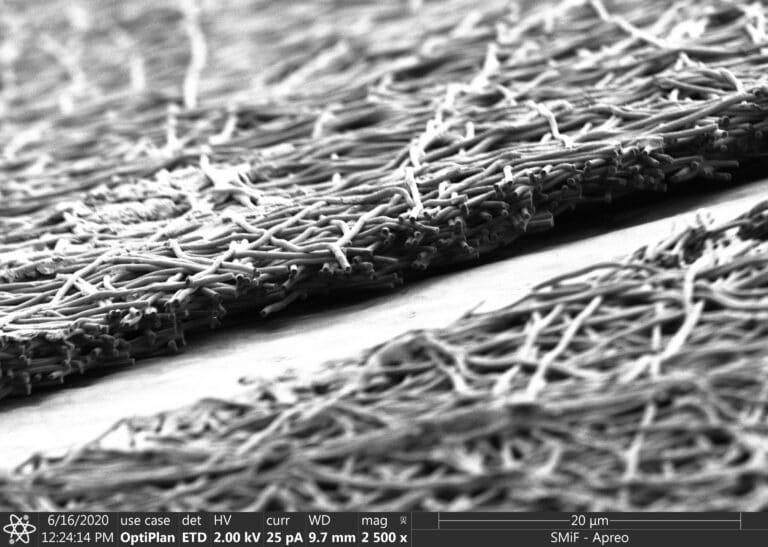
Samira Musah, an assistant professor of biomedical engineering and medicine at Duke, and George (Xingrui) Mou, a PhD student in Musah’s lab and first author on the paper, turned to silk fibroin, a protein created by silkworms that can be electronically spun into a membrane. The porous material, reminiscent of spaghetti or a Jackson Pollock painting under a microscope, better mimics the structure of the extracellular matrix found in human organs.
“The silk fibroin allowed us to bring the membrane thickness down from 50 microns to five or fewer, which gets us an order of magnitude closer to what you’d see in a living organism,” explained Mao.
Advancing Kidney Disease Modeling and Drug Screening
To test the new membrane, Musah and Mao applied the material to their kidney chip models, which resemble a cross-section of a human kidney, specifically the glomerular capillary wall. After adding human induced pluripotent stem cell derivatives into the chip, they observed that the cells were able to send signals across the ultrathin membrane, facilitating differentiation into glomerular cells, podocytes, and vascular endothelial cells. The platform also triggered the development of endothelial fenestrations, holes that allow for the passage of fluid between the cellular layers.
“The new microfluidic chip system’s ability to simulate in vivo-like tissue-tissue interfaces and induce the formation of specialized cells, such as fenestrated endothelium and mature glomerular podocytes from stem cells, holds significant potential for advancing our understanding of human organ development, disease progression, and therapeutic development,” said Musah.
Despite affecting more than 15 percent of American adults, researchers lack effective models for kidney disease. Patients are often not diagnosed until substantial damage has occurred, requiring dialysis or kidney transplantation. Musah and colleagues hope to use this technology to better understand the mechanisms behind kidney disease and discover new biomarkers and drug candidates.
“Using this platform to develop kidney disease models could help us discover new biomarkers of the disease,” said Mao. “This could also be used to help us screen for drug candidates for several kidney disease models. The possibilities are very exciting.”
The implications of this technology extend far beyond kidney disease modeling. “This technology has implications for all organ-on-a-chip models,” said Musah. “Our tissues are made up of membranes and interfaces, so you can imagine using this membrane to improve models of other organs, like the brain, liver, and lungs, or other disease states. That’s where the power of our platform really lies.”
As researchers continue to refine and apply this groundbreaking technology, the potential for advancing our understanding of human organ development, disease progression, and therapeutic development is immense. The ultrathin silk membrane developed by Musah and her team at Duke University represents a significant leap forward in the field of organ-on-a-chip technology, offering a powerful tool for unlocking the secrets of human biology and revolutionizing the way we approach disease modeling and drug discovery.