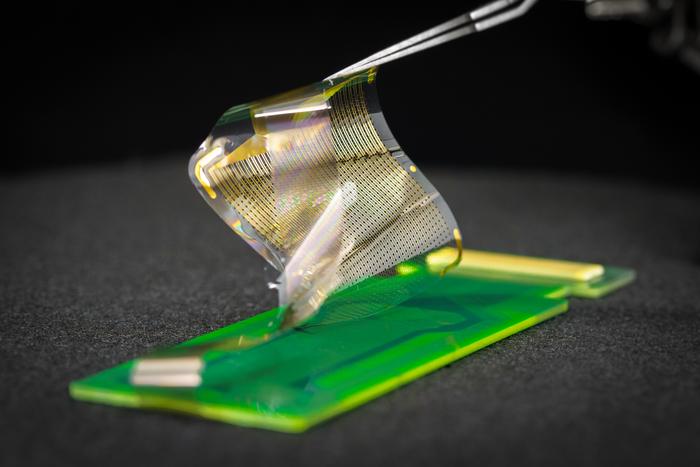
A new device developed by engineers at the University of California San Diego has received FDA approval for a clinical trial to test its effectiveness in recording brain activity during surgery. The device, called the platinum nanorod grid (PtNRGrid), features a densely packed grid of 1,024 embedded electrocorticography (ECoG) sensors that can record electrical signals directly from the surface of the human brain in unprecedented detail.
The PtNRGrid’s breakthrough resolution could provide better guidance for planning and performing surgeries to remove brain tumors and treat drug-resistant epilepsy. By recording brain signals with higher resolution, the device could improve neurosurgeons’ ability to minimize damage to healthy brain tissue and precisely identify the regions of the brain where epileptic seizures originate.
Ultra-Thin, Flexible, and High-Density Sensor Grid
The PtNRGrid, invented by Professor Shadi Dayeh and his team at UC San Diego, is approximately 6 microns thin–smaller than one-tenth of a human hair–and flexible. This allows the device to adhere and conform to the surface of the brain, bending as the brain moves while providing high-quality, high-resolution recordings of brain activity. In contrast, the ECoG grids most commonly used in surgeries today typically have between 16 and 64 sensors, are rigid, stiffer, and more than 0.5 mm in thickness, and do not conform to the curved surface of the brain.
“This accomplishment ushers in a new era of clinical neuroscience and neuromonitoring,” Dayeh said. “We are very excited to receive the FDA approval to apply our groundbreaking PtNRGrid in a clinical setting. It is a credit to the hard work of my team members who worked tirelessly to meet the quality criteria mandated by the FDA.”
Collaboration with Leading Medical Institutions
The clinical trial, designed to demonstrate the effectiveness of the PtNRGrid device in mapping both normal and pathological brain activity, will involve partnerships with clinician-scientists from UC San Diego, Massachusetts General Hospital (MGH), and Oregon Health & Science University (OHSU). In the first phase, surgeons will implant the PtNRGrid in 20 patients and compare its performance with the current state-of-the-art.
The team has already demonstrated the PtNRGrid’s ability to map motor and language brain activity, as well as epileptic discharges, by producing panoramic videos of brain waves over 10 square centimeters of the brain’s cortex while maintaining resolution at a microscopic level. Pending the success of this staged trial, the team will work towards making the PtNRGrid available for commercial use at scale.
The potential applications of the PtNRGrid extend beyond surgical guidance, as demonstrating that ECoG grids with thousands of sensors can record brain activity with high fidelity opens new opportunities in neuroscience for uncovering a deeper understanding of how the human brain functions. This, in turn, could lead to improved treatments grounded in an enhanced understanding of brain function.
Headline: FDA Greenlights Clinical Trial for High-Res Brain Activity Recording Device, Set to Revolutionize Neurosurgery