Researchers have developed a groundbreaking chemical reaction that significantly improves the production of adenosine diphosphate (ADP) and adenosine triphosphate (ATP)-containing molecules. This advancement could accelerate our understanding of cellular energy processes and pave the way for new therapeutic approaches.
Overcoming Traditional Synthesis Challenges
ADP and ATP are vital molecules in cellular energy transfer and metabolism. However, creating synthetic versions of these compounds has long been a stumbling block for researchers. Traditional methods often result in low yields, poor reproducibility, and are limited in the types of molecules they can accommodate.
Hide-Nori Tanaka, associate professor at the Institute for Glyco-core Research (iGCORE) and Gifu University, explains the core issue: “The problem with traditional coupling reactions is the lack of reproducibility in chemical synthesis of ADP- and ATP-containing molecules, which are necessary for molecular-level studies to elucidate their detailed biological functions via conventional methods. This lack of reproducibility is due to the water content in highly polar phosphate-containing substrates.”
The research team, led by Tanaka, developed a novel approach that addresses these longstanding challenges. Their method modifies a coupling reaction to produce ADP and ATP-containing molecules, including important nicotinamide adenine dinucleotide (NAD+) analogs, with significantly higher yields and consistency.
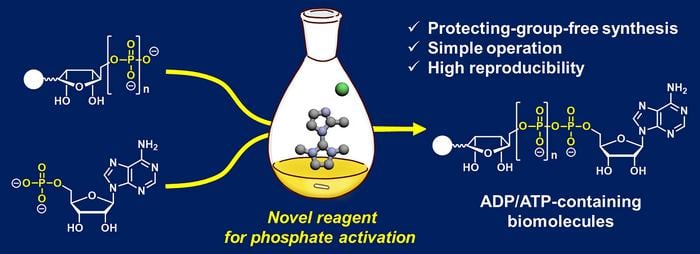
A Water-Resistant Solution
The key to the team’s success lies in their creation of a water-resistant phosphate-activation reagent. By modifying an existing compound called ImIm-Cl, they produced 2-MeImIm-Cl, which remains stable in the presence of water. This seemingly small change had a dramatic impact on the reaction’s efficiency.
“To address [the synthesis] problem, we developed an efficient and reliable method enabling reproducible and high-[yield] access to ADP- and ATP-containing molecules through [a] protecting-group-free reaction using a hydrolysis-stable reagent for phosphate activation,” Tanaka stated.
The results were impressive. Using their optimized coupling reactions, the team achieved yields of 55-75% for ADP-ribose derivatives, 53-84% for NAD+ analogs, and 46-67% for ATP formations. Even larger molecular couplings, such as ADP-ribosyl peptides, reached yields of 65-70%.
These numbers represent a significant improvement over traditional methods, which often struggle to achieve consistent yields above 50%. Moreover, the new technique eliminates the need for complex protecting groups, simplifying the overall process.
Why it matters: This advancement could accelerate research into cellular energy processes, potentially leading to new treatments for metabolic disorders and a deeper understanding of fundamental biology. The ability to produce these crucial molecules more efficiently and in greater quantities opens up new avenues for drug discovery and biotechnology applications.
Looking ahead, Tanaka and his team plan to apply this method to synthesize an even wider range of ADP and ATP-containing biomolecules. “Our ultimate goal is to elucidate their detailed functions [with a] chemical biology approach using synthetic molecules,” Tanaka explained.
As researchers continue to explore the potential of this new synthesis method, we may see rapid advancements in our understanding of cellular energy dynamics and the development of novel therapeutic strategies targeting these crucial molecular processes.