Estimated reading time: 5 minutes
A new study has uncovered a crucial molecular mechanism that connects aging with the development of neurodegenerative diseases like Alzheimer’s and Parkinson’s. Researchers from Tokyo Medical and Dental University (TMDU) in Japan have identified a key protein that helps maintain the structure of cell nuclei, potentially opening new avenues for treating age-related brain disorders.
The research, published in The EMBO Journal on August 5, 2024, focuses on a protein called polyglutamine binding protein 3 (PQBP3). This protein plays a vital role in preserving the integrity of the nuclear membrane – the barrier that protects a cell’s genetic material.
Why it matters: Understanding the molecular processes that link aging to neurodegenerative diseases could lead to new treatments that address both brain aging and neurodegeneration simultaneously.
Unraveling the PQBP3 Mystery
Professor Hitoshi Okazawa and his team at TMDU have been studying the PQBP family of proteins for over two decades. Their latest work reveals how PQBP3 behaves differently in young versus aging cells.
In healthy cells, PQBP3 is typically found near the nucleolus – a structure within the cell nucleus. However, the researchers observed that in senescent (aging) cells, PQBP3 moves away from its usual location.
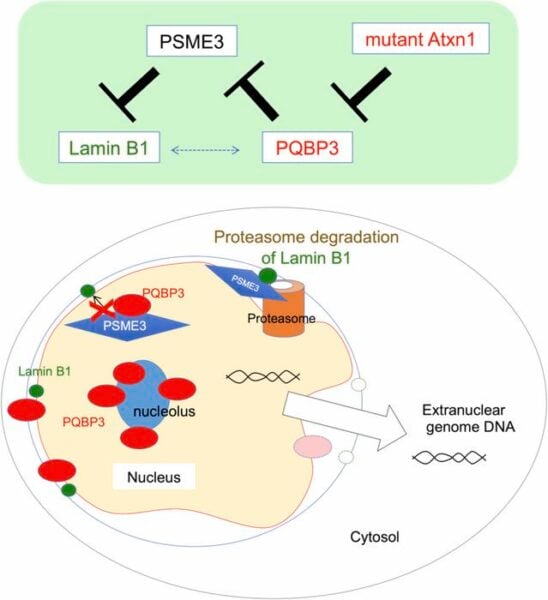
“During senescence, PQBP3 relocated from the nucleolus to the nucleoplasm or cytoplasm,” explains Professor Okazawa. “This relocation was accompanied by leakage of nuclear DNA into the cytoplasm.”
The Nuclear Membrane Connection
Using advanced microscopy techniques, the team discovered that cells with displaced PQBP3 had unstable nuclear membranes. Further investigation revealed the molecular mechanism behind this instability.
PQBP3 normally binds to another protein called PSME3, which is responsible for breaking down various cellular components. By attaching to PSME3, PQBP3 prevents it from degrading a crucial structural protein in the nuclear membrane called Lamin B1.
In aging cells, however, there’s less PQBP3 available to perform this protective function. As a result, Lamin B1 gets broken down more quickly, weakening the nuclear membrane.
From Aging to Neurodegeneration
To connect these findings to brain diseases, the researchers examined cells and mouse models of spinocerebellar ataxia type 1 (SCA1), a neurodegenerative disorder. They found that in SCA1, abnormal protein clumps “capture” PQBP3, preventing it from stabilizing the nuclear membrane.
This discovery suggests a common thread between normal aging and the accelerated cellular breakdown seen in neurodegenerative diseases.
A Double-Edged Sword
While targeting PQBP3 could potentially address both brain aging and neurodegeneration, Professor Okazawa urges caution: “PQBP3 may be a double-edged sword, as it is involved in a fundamental biological problem of contrasting cellular pathologies, namely cancer and neurodegeneration.”
The protein’s role in cellular senescence might help suppress cancer in non-neuronal cells, highlighting the complexity of developing treatments based on this research.
As scientists continue to unravel the intricate relationships between aging, cellular health, and brain diseases, this study provides a valuable piece of the puzzle. The insights gained from understanding PQBP3’s function could lead to more targeted and effective therapies for age-related neurodegenerative disorders in the future.
Quiz:
- What is the main function of PQBP3 in healthy cells?
- How does PQBP3’s behavior change in senescent (aging) cells?
- Why might targeting PQBP3 be considered a “double-edged sword” in potential treatments?
Answer Key:
- PQBP3 helps maintain the integrity of the nuclear membrane by preventing the degradation of Lamin B1.
- In senescent cells, PQBP3 relocates from the nucleolus to the nucleoplasm or cytoplasm, leading to nuclear membrane instability.
- While targeting PQBP3 could potentially address brain aging and neurodegeneration, it might also increase cancer risk in non-neuronal cells due to its role in cellular senescence.